Publications
Recent advances in the synthesis of extensive libraries of heparan sulfate oligosaccharides for structure-activity relationship studies
Ramadan, S.; Mayieka, M.; Pohl N. L. B.; Liu, J.; Hsieh-Wilson, L. C.; Huang, X.* Curr. Opin. Chem. Biol. 2024, 80, 102455.DOI: 10.1016/j.cbpa.2024.102455
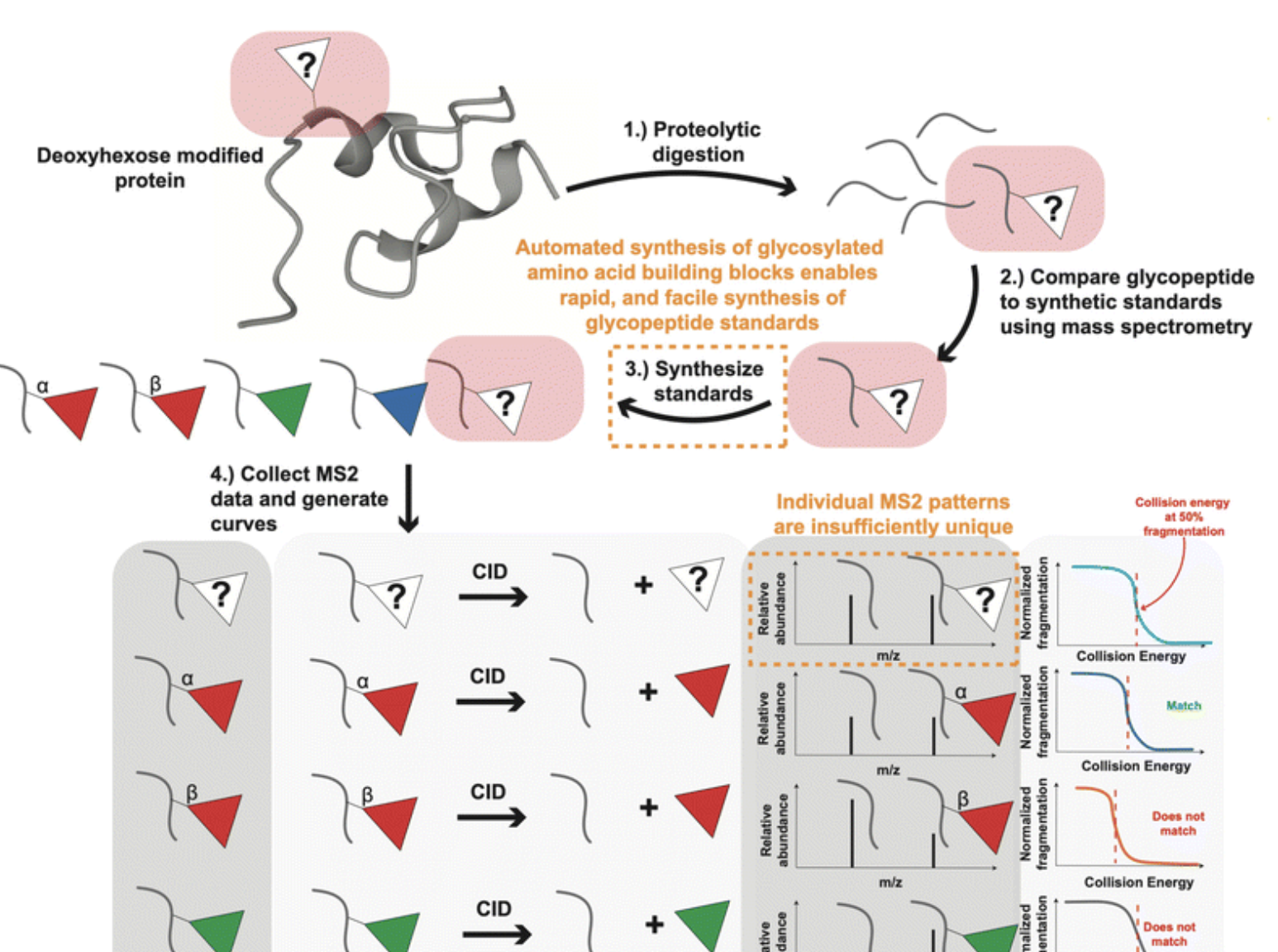
An identification method to distinguish monomeric sugar isomers on glycopeptides
DeYong, A. E.; Trinidad, J. C.*; Pohl, N. L. B.* An identification method to distinguish monomeric sugar isomers on glycopeptides. Analyst. 2023, 148, 4438-4446.DOI: 10.1039/D3AN01036H
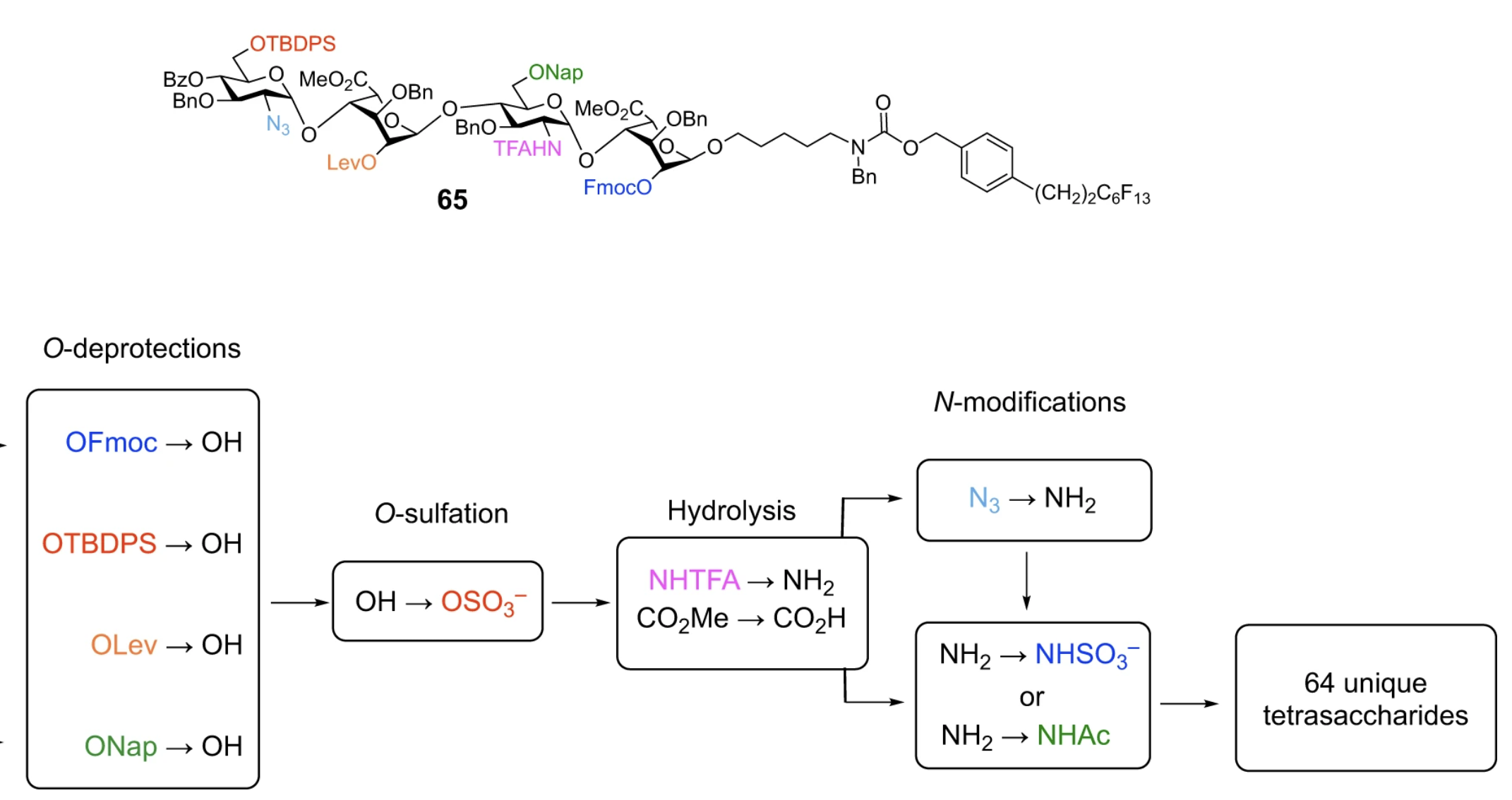
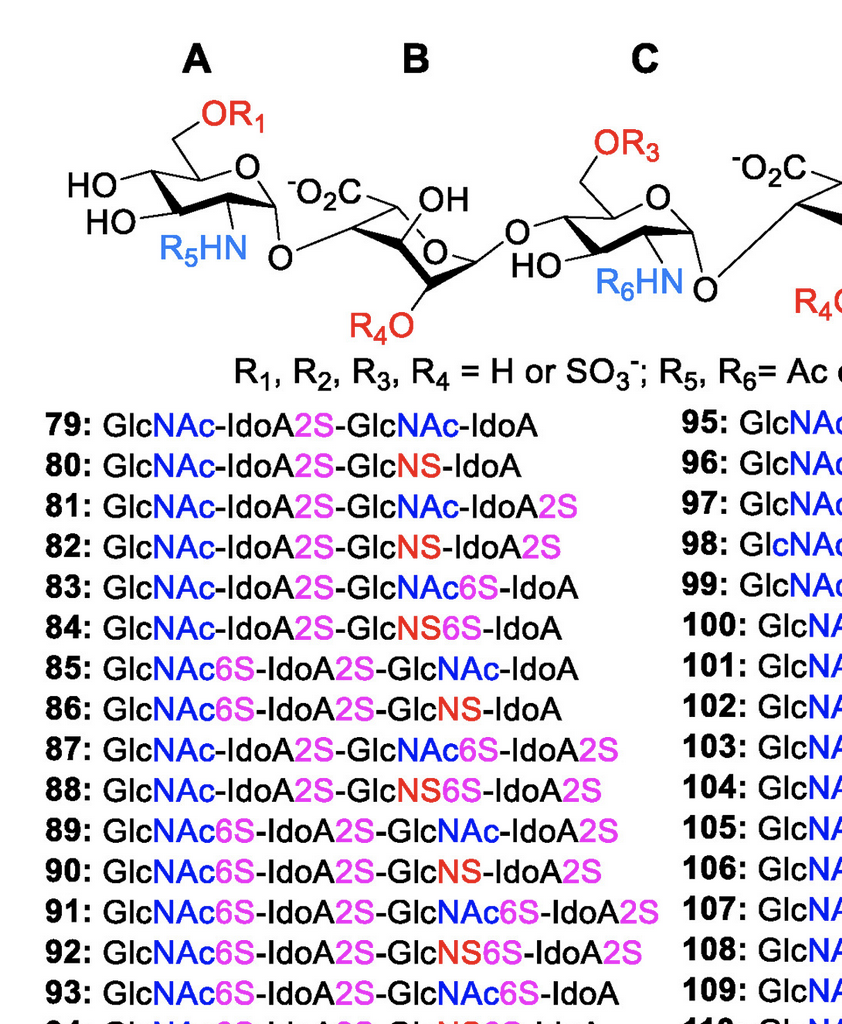
Efficient Platform for Synthesizing Comprehensive Heparan Sulfate Oligosaccharide Libraries for Decoding Glycosaminoglycan-Protein Interactions
Wang, L.; Sorum, A. W.; Huang, B.-S.; Kern, M. K.; Su, G.; Pawar, N.; Huang, X.; Liu, J.; Pohl, N. L. B.; Hsieh-Wilson, L. C.* Efficient Platform for Synthesizing Comprehensive Heparan Sulfate Oligosaccharide Libraries for Decoding Glycosaminoglycan-Protein Interactions. Nat. Chem. 2023, 15, 1108-1117. DOI: 10.1038/s41557-023-01248-4

Teach Better, Save Time, and Have More Fun: A Guide to Teaching and Mentoring in Science
Beuning, P. J.; Besson, D. Z.; Snyder, S. A.; Pohl, N. L. B. Teach Better, Save Time, and Have More Fun: A Guide to Teaching and Mentoring in Science. 2nd ed. with an annotated bibliography by Salgado, I. D. and Hotchkiss, A. B. Research Corporation for the Advancement of Science, 2022, ISBN 978-0-692-26580-2
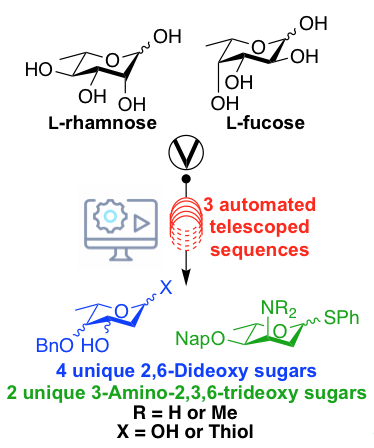
Automated, multistep continuous-flow synthesis of 2,6-dideoxy and 3-amino-2,3,6-trideoxy monosaccharide building blocks
Yalamanchili, S.; Nguyen, T.V.; Zsikla, A.; Stamper, G.; DeYong, A. E.; Florek, J.; Vasquez, O.; Pohl, N. L. B.*; Bennett, C. S.* Automated, multistep continuous-flow synthesis of 2,6-dideoxy and 3-amino-2,3,6-trideoxy monosaccharide building blocks. Angew. Chem. Int. Ed. 2021, 60, 23171-23175. DOI: 10.1002/anie.202109887
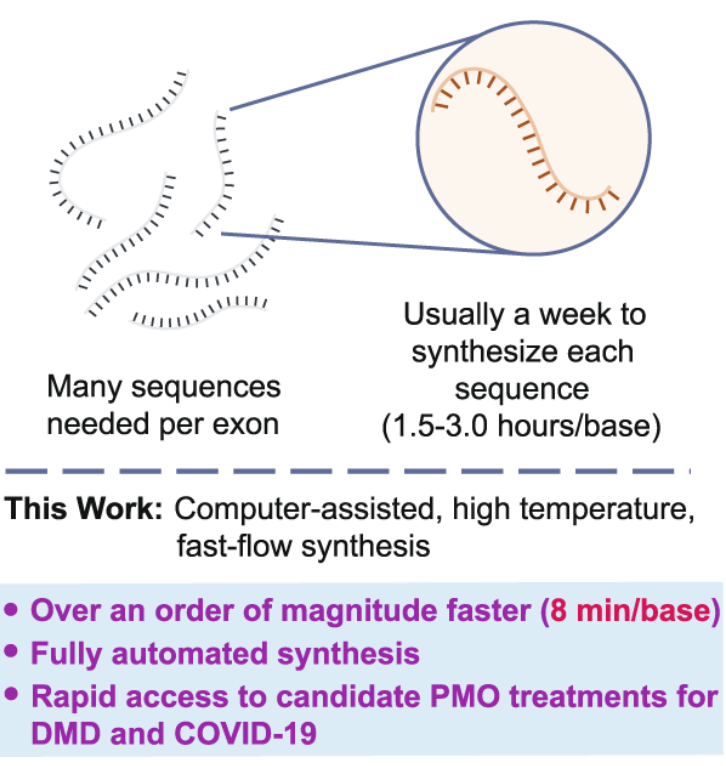
Fully automated fast-flow synthesis of antisense phosphorodiamidate morpholino oligomers
Li, C.; Callahan, A. J.; Simon, M. D.; Totaro, K. A.; Mijalis, A. J.; Phadke, K.-S.; Zhang, G.; Hartrampf, N.; Schissel, C. K.; Zhou, M.; Zong, H.; Hanson, G. J.; Laos, A.; Pohl, N. L. B.; Verhoeven, D. E.; Pentelute, B. L.* Fully automated fast-flow synthesis of antisense phosphorodiamidate morpholino oligomers. Nat. Commun. 2021, 12, 4396. DOI: 10.1038/s41467-021-24598-4
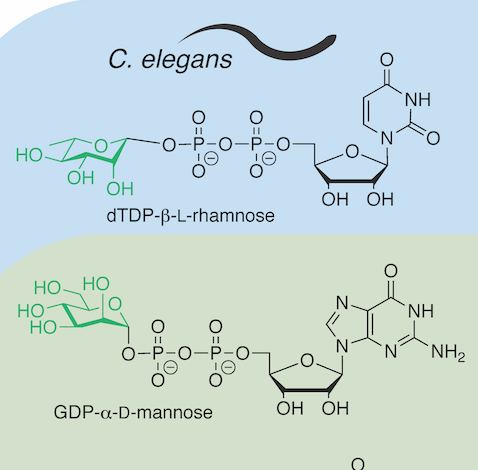
Glycobiology of Caenorhabditis elegans
Paschinger, K.; Yan, S.; Pohl, Nicola L. B.; Wilson, Iain B. H. Glycobiology of Caenorhabditis elegans. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, DOI: 10.1016/B978-0-12-819475-1.00071-7. Comprehensive Glycosciences, 2nd edition, Elsevier, 2021, ISBN-13: 9780128194571.
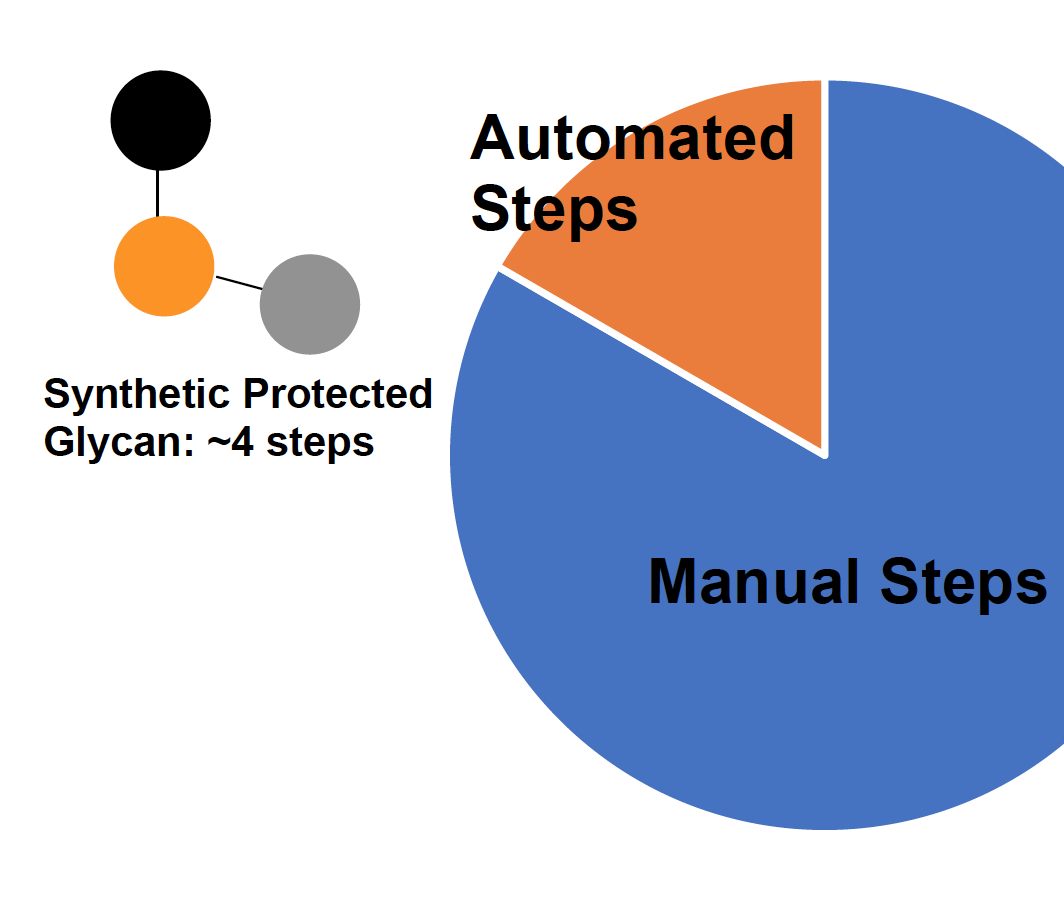
Synthesis of Carbohydrate Building Blocks for Automated Oligosaccharide Synthesis
DeYong, A.; Rudich, Meredith L.; Pohl, Nicola L. B. Synthesis of Carbohydrate Building Blocks for Automated Oligosaccharide Synthesis. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, DOI: 10.1016/B978-0-12-819475-1.00109-7. Comprehensive Glycosciences, 2nd edition, Elsevier, 2021, ISBN-13: 9780128194571.
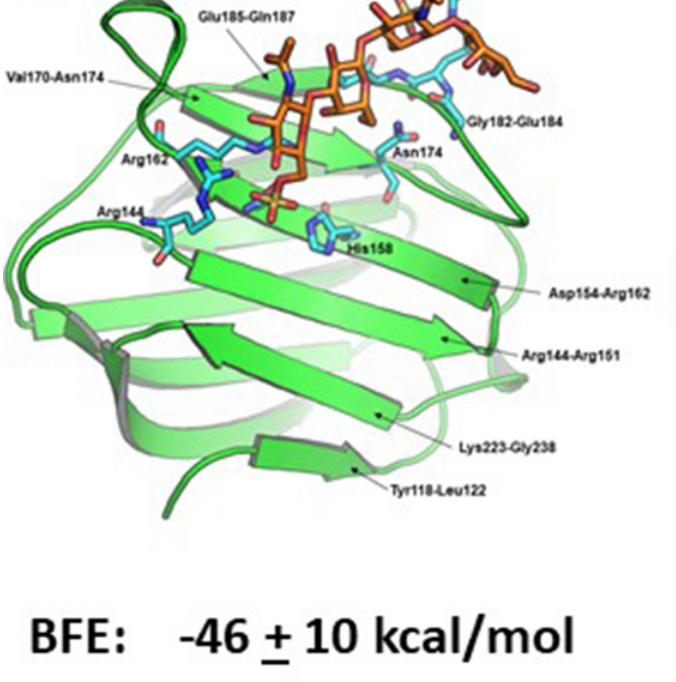
Structural insight into the binding of human galectins to corneal keratan sulfate, its desulfated form and related saccharides.
Miller, M. C.; Cai, C.; Wichapong, K.; Bhaduri, S.; Pohl, N. L. B.; Lindhardt, R. J.; Gabius, H. J.*; Mayo, K. H.* Structural insight into the binding of human galectins to corneal keratan sulfate, its desulfated form and related saccharides. Sci. Rep. 2020, 10, 15708.DOI:10.1038/s41598-020-72645-9

A Very Short History of the Carbohydrate Division of the American Chemical Society
Lowary, T. L.; Pohl, N. L. A Very Short History of the Carbohydrate Division of the American Chemical Society. J. Org. Chem. 2020, 85, 15778-15779. DOI: 10.1021/acs.joc.0c02753

A New Era of Discovery in Carbohydrate Chemistry
Li, X.; Lowary, T.; Driguez, P.A.; Pohl, N. L.; Zhu J. J. Org. Chem. 2020, 85, 15770-15772. DOI:10.1021/acs.joc.0c02548

Book review: Etymology of the elements
Pohl, N. L. B.* Science, 2020, 367, 860. DOI:10.1126/science.aba3488
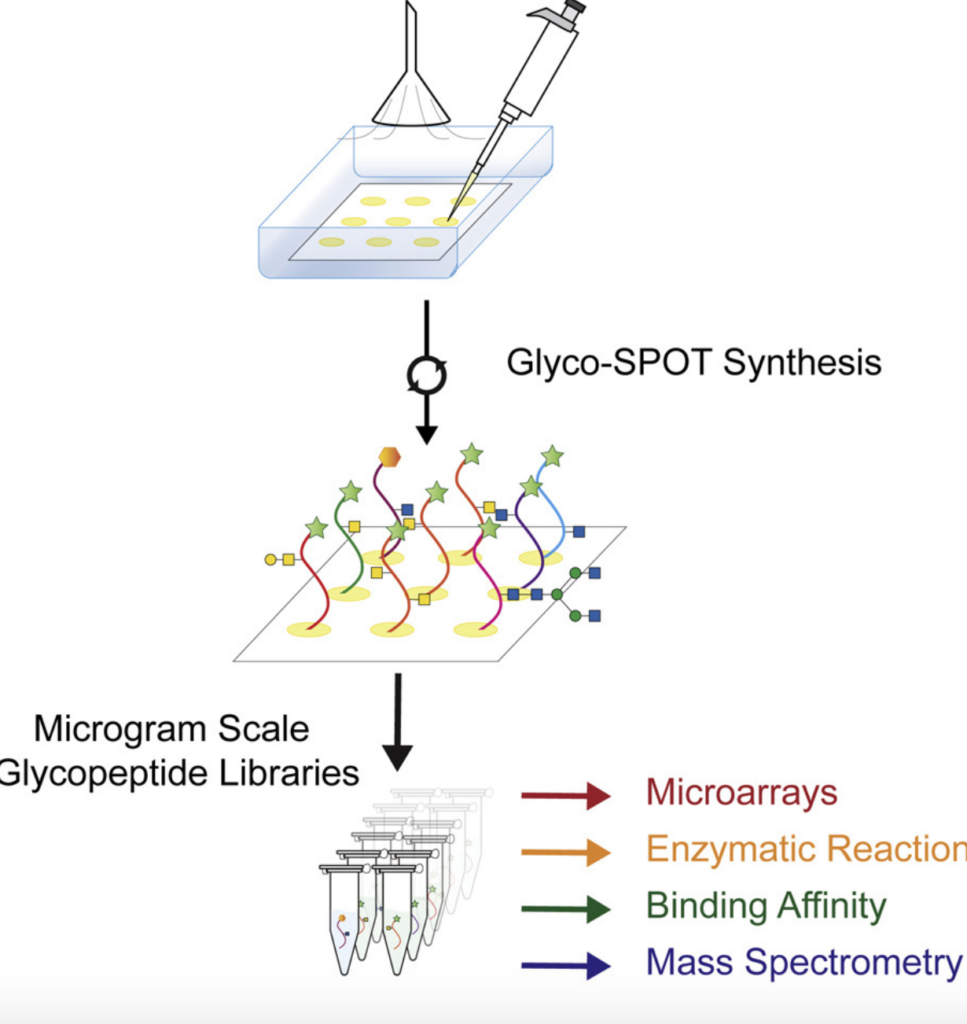
Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries
Mehta, A. Y.; Veeraiah, R. K. H.; Dutta, S.; Goth, C. K.; Hanes, M. S.; Gao, C.; Stavenhagen, K.; Kardish, R.; Matsumoto, Y.; Heimburg-Molinaro, J.; Boyce, M.; Pohl, N. L. B.*; Cummings, R. D.* Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries. Cell Chem. Biol. 2020, 27, 1207-1219.e9.DOI:10.1016/j.chembiol.2020.06.007
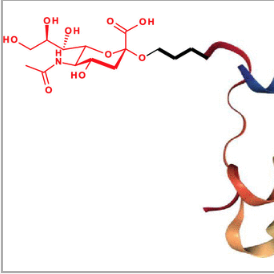
Addition of sialic acid to insulin confers superior physical properties and bioequivalence
Kabotso, D.; Smiley, D.; Mayer, J.; Gelfanov, V.; Perez-Tilve, D.; DiMarchi, R.; Pohl, N. L. B.*; Liu, F.* Addition of sialic acid to insulin confers superior physical properties and bioequivalence. J. Med. Chem. 2020, 63, 6143. DOI:10.1021/acs.jmedchem.0c00266
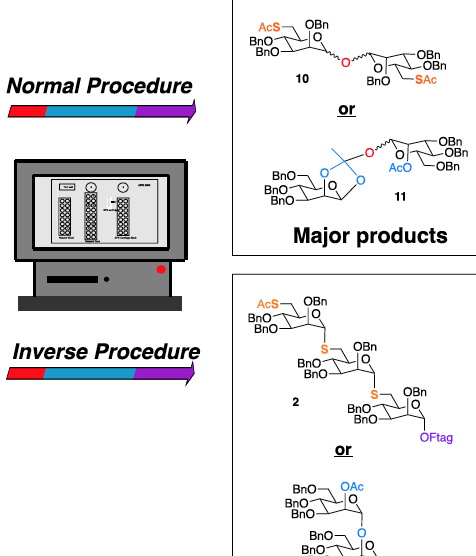
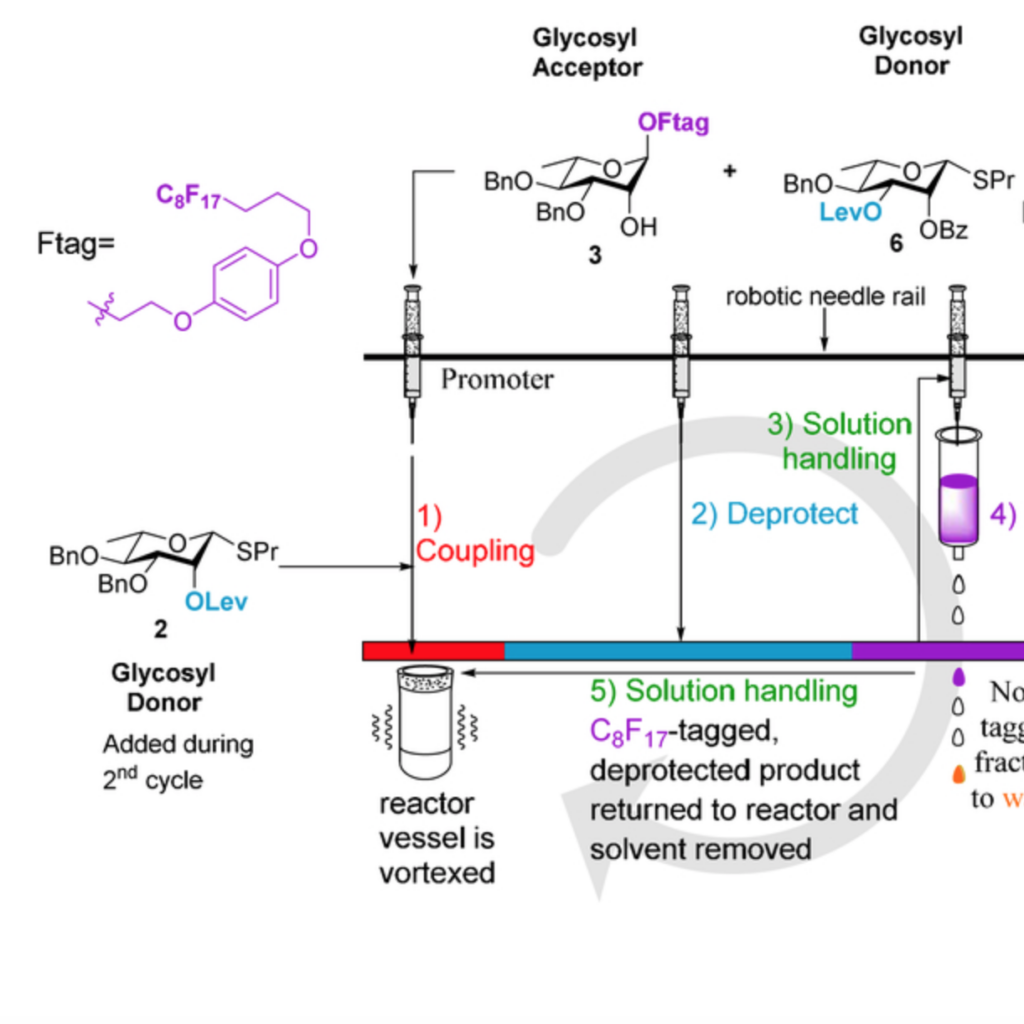
Automated solution-phase synthesis of S-glycosides for the production of oligomannopyranoside derivatives
Kern, M.; Pohl, N. L. B.* Automated solution-phase synthesis of S-glycosides for the production of oligomannopyranoside derivatives. Org. Lett. 2020, 22, 4156. DOI:10.1021/acs.orglett.0c01236
Automated solution-phase synthesis of alpha-1-2, 1-3-type rhamnans and rhamnan sulfate fragments.
Kohout, V. R.; Pohl, N. L. B.* Automated solution-phase synthesis of alpha-1-2, 1-3-type rhamnans and rhamnan sulfate fragments. Carbohydr. Res. 2019, 486, 107829. DOI:10.1016/j.carres.2019.107829
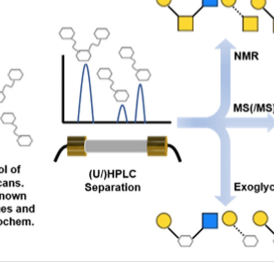
Advancing solutions to the carbohydrate sequencing challenge
Gray, C. J.; Migas, L. G.; Barran, P. E.; Pagel, K.; Seeberger, P. H.; Eyers, C. E.; Boons, G.-J.; Pohl, N. L. B.; Compagnon, I.; Widmalm, G.; Flitsch, S. L.* Advancing solutions to the carbohydrate sequencing challenge. J. Am. Chem. Soc. 2019, 141, 14463-14479. (Perspective).
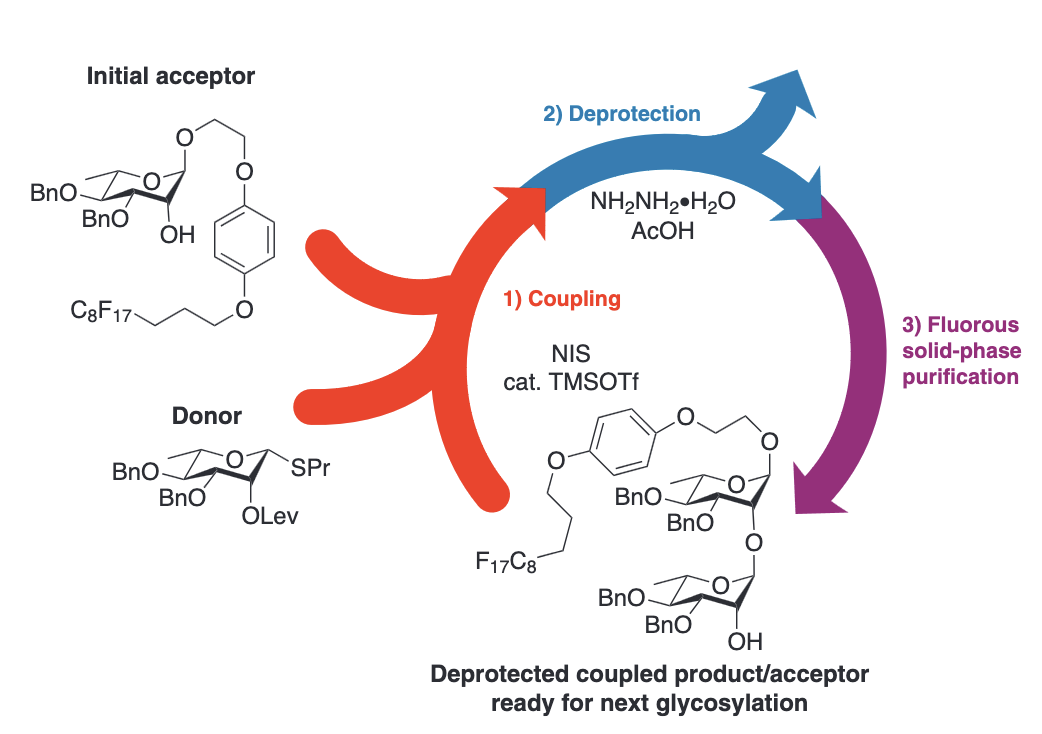
Acid-mediated N-iodosuccinmide-based thioglycoside activation for the automated solution-phase synthesis of alpha-1,2-linked-rhamnopyranosides
Kohout, V. R.; Pirinelli, A. L.; Pohl, N. L. B.* Acid-mediated N-iodosuccinmide-based thioglycoside activation for the automated solution-phase synthesis of alpha-1,2-linked-rhamnopyranosides. Pure Appl. Chem. 2019, 91, 1243.DOI: 10.1515/pac-2019-0307
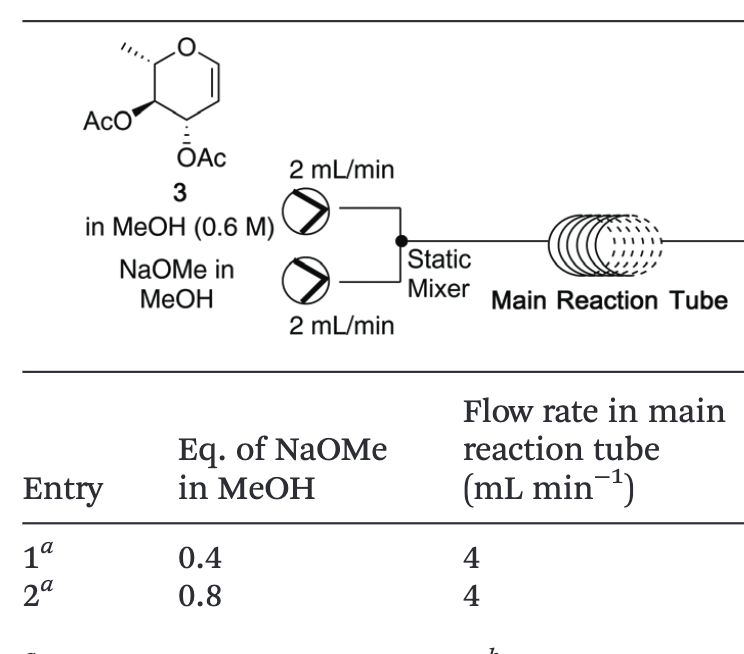
Modular continuous flow synthesis of orthogonally protected 6-deoxy glucose glycals
Yalamanchili, S.; Nguyen, T.V.; Pohl, N. L. B.*; Bennett, C. S.* Modular continuous flow synthesis of orthogonally protected 6-deoxy glucose glycals. Org. Biomol. Chem. 2020, 18, 3254. DOI: 10.1039/D0OB00522C
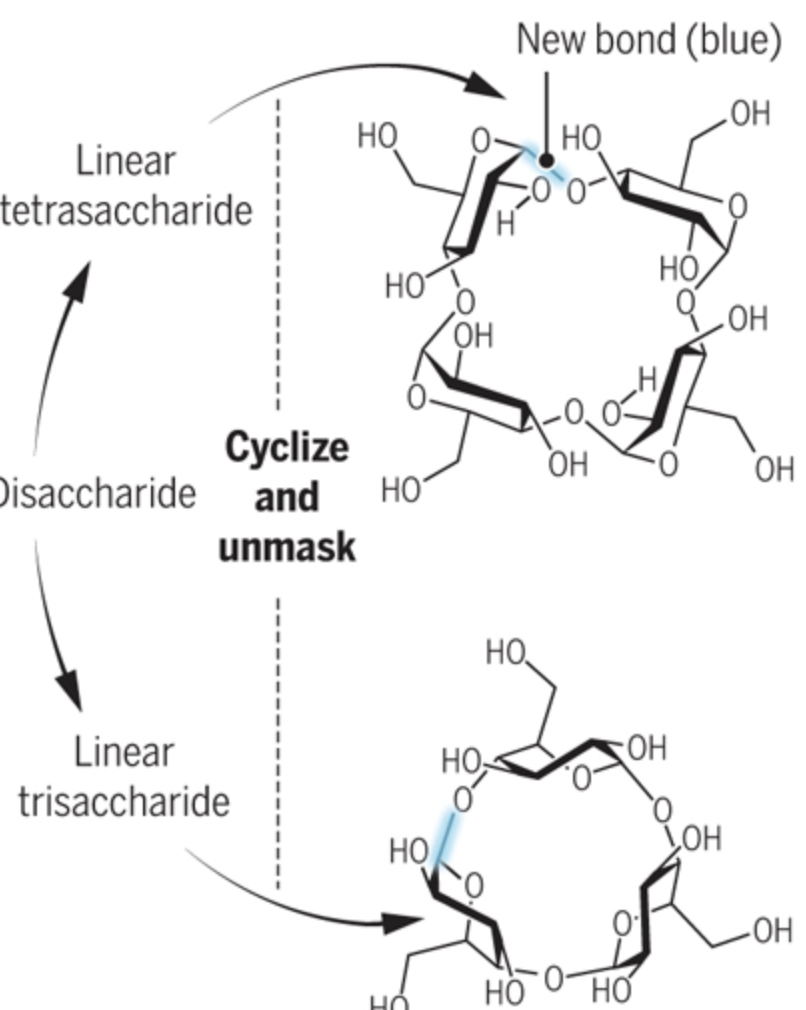
Putting Sugars Under Strain
Pohl, N. L. B.* Putting Sugars Under Strain. Science, 2019, 364, 631. (invited commentary) DOI: 10.1126/science.aax3501

Book review: A Grand Story of Carbon
Pohl, N. L. B.* A Grand Story of Carbon. Science, 2019, 365, 37. DOI: 10.1126/science.aaw7726
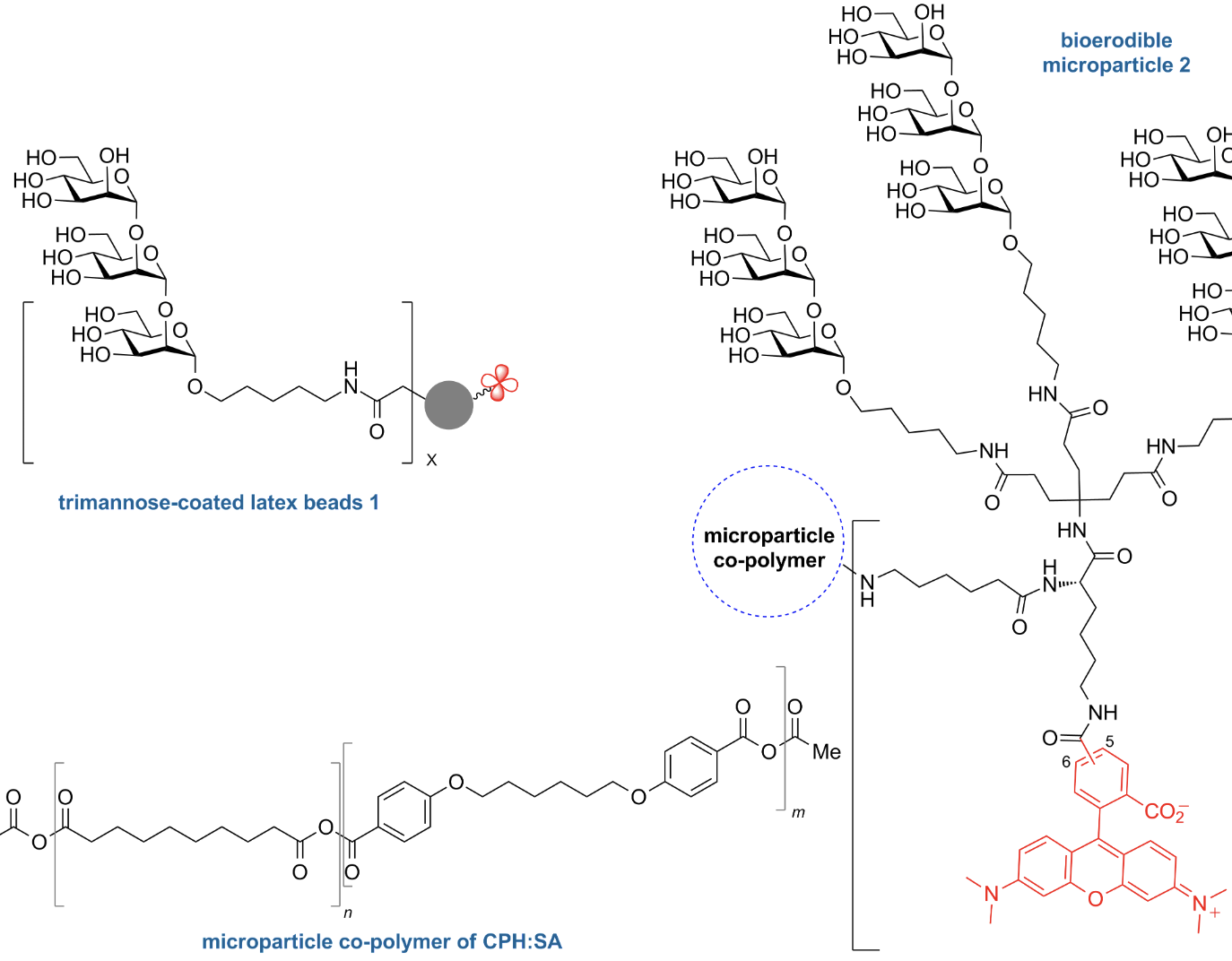
Design and synthesis of multivalent α-1,2-trimannose-linked bioerodible microparticles for applications in immune response studies of Leishmania major infection
Rintelmann, C. L.; Grinnage-Pulley, T. L.; Ross, K.; Kabotso, D. E. K.; Toepp, A.; Cowell, A.; Petersen, C. A.; Narasimhan, B.; Pohl, N. L. B.* Design and synthesis of multivalent α-1,2-trimannose-linked bioerodible microparticles for applications in immune response studies of Leishmania major infection. Beilstein J. Org. Chem. 2019, 15, 623. DOI: 10.3762/bjoc.15.58
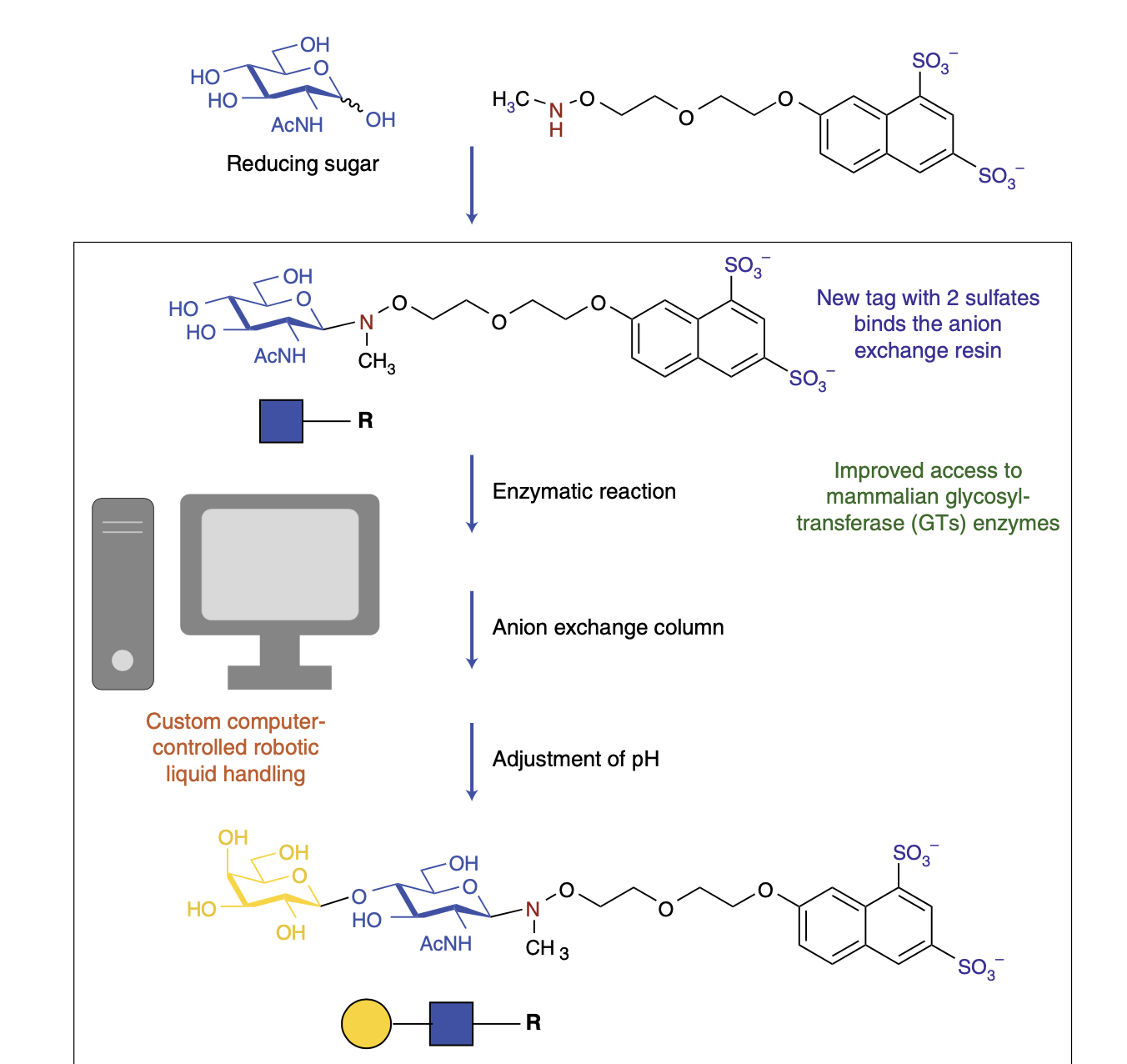
Robots Command Enzymes
Pohl, N. L. B.* Robots Command Enzymes. Nature Chem. 2019, 11, 201. (invited commentary) DOI: 10.1038/s41557-019-0228-7

Book review: Open Minds
Pohl, N. L. B.* Open Minds. Science, 2018, 360, 610. DOI: 10.1126/science.aat4324
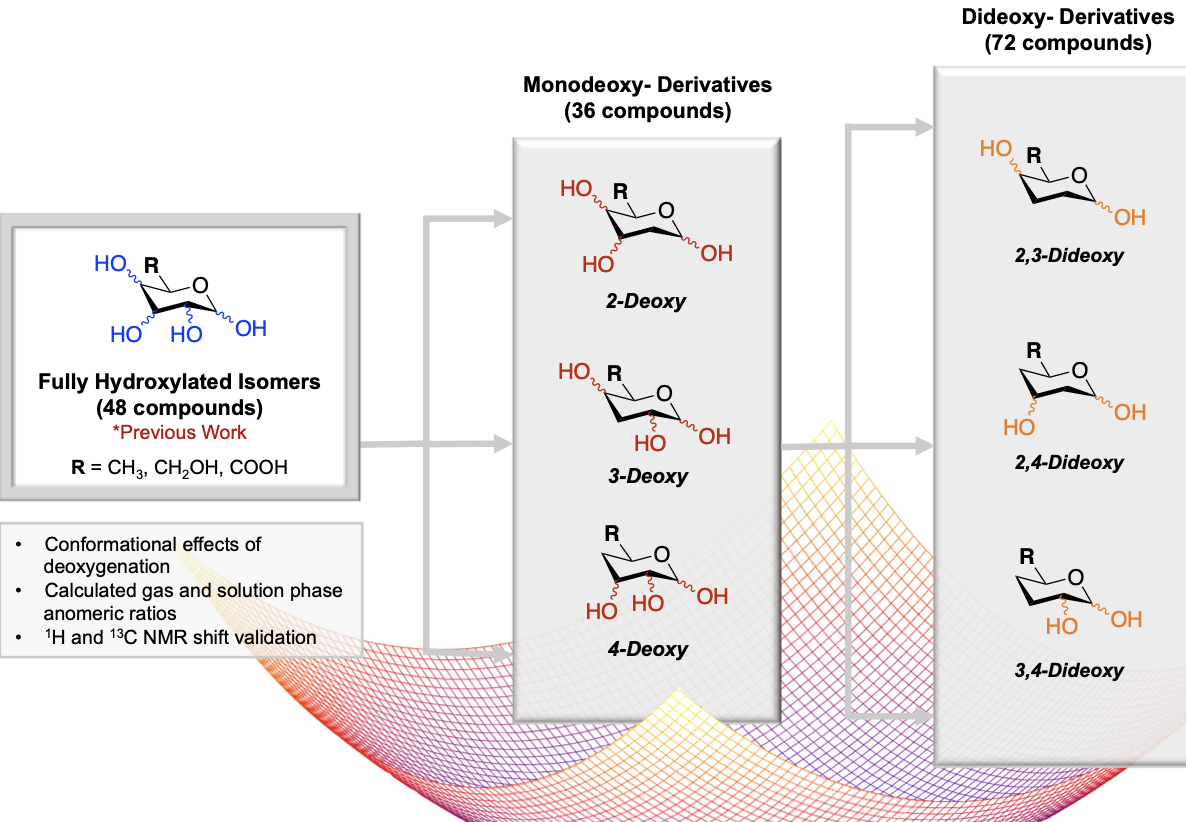
Probing Deoxysugar conformational preference: A comprehensive computational study investigating the effects of deoxygenation
Vickman, A.; Pohl, N. L. B.* Probing Deoxysugar conformational preference: A comprehensive computational study investigating the effects of deoxygenation. Carbohydr. Res. 2019, 475, 17. DOI: 10.1016/j.carres.2018.12.003
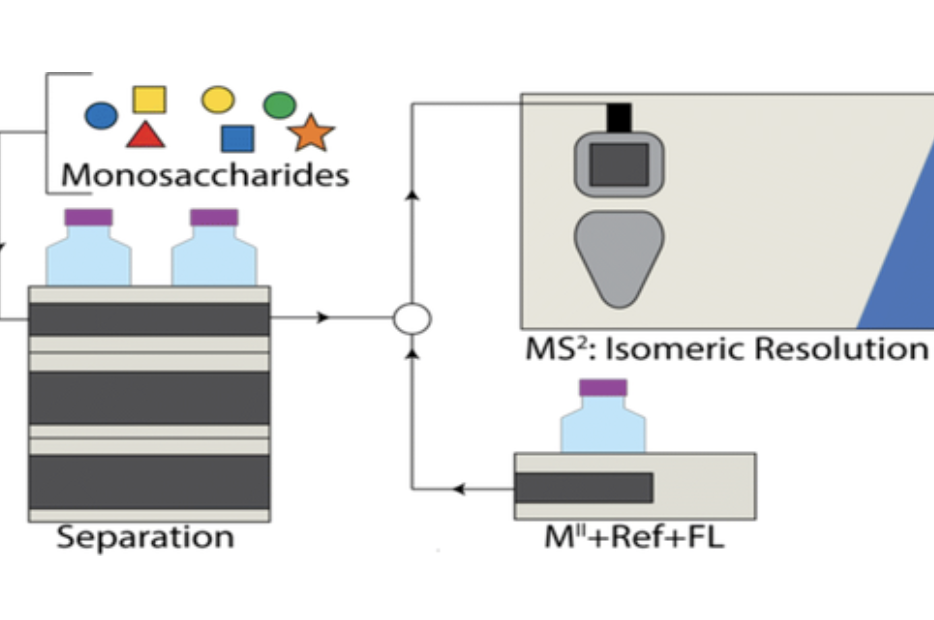
Development of a post-column liquid chromatographic chiral addition method for the separation and resolution of common mammalian monosaccharides
Wooke, Z.; Nagy, G.; Barnes, L. F.; Pohl, N. L. B.* Development of a post-column liquid chromatographic chiral addition method for the separation and resolution of common mammalian monosaccharides. J. Am. Soc. Mass Spectrom. 2019, 30, 419. DOI: 10.1007/s13361-018-2095-7
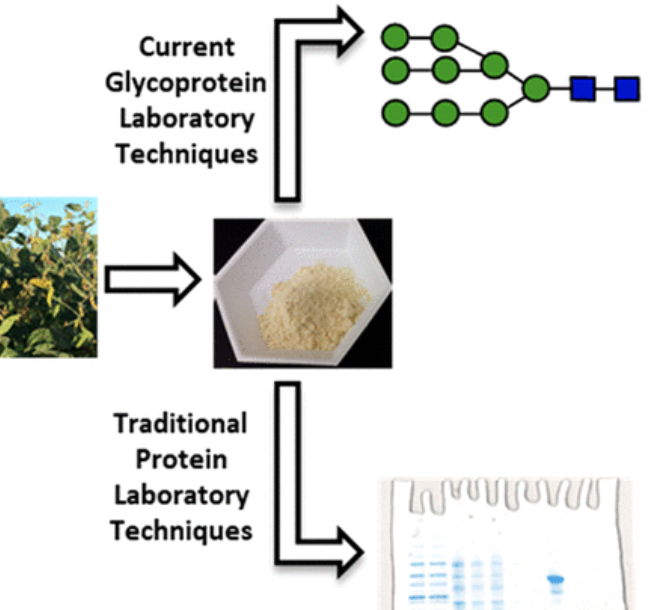
Protein N-glycans: Incorporating glycochemistry into the undergraduate laboratory curriculum
Kohout, V. R.; Wooke, Z. J.; McKee, A. G.; Thielges, M. C.; Robinson, J. K.*; Pohl, N. L. B.* Protein N-glycans: Incorporating glycochemistry into the undergraduate laboratory curriculum. J. Chem. Educ. 2018, 95, 2249. DOI: 10.1021/acs.jchemed.8b00539

Introduction: Carbohydrate Chemistry
Pohl, N. L. B.* Introduction: Carbohydrate Chemistry. Chem. Rev. 2018, 118, 7865. (invited guest editor/commentary on special issue) DOI: 10.1021/acs.chemrev.8b00512
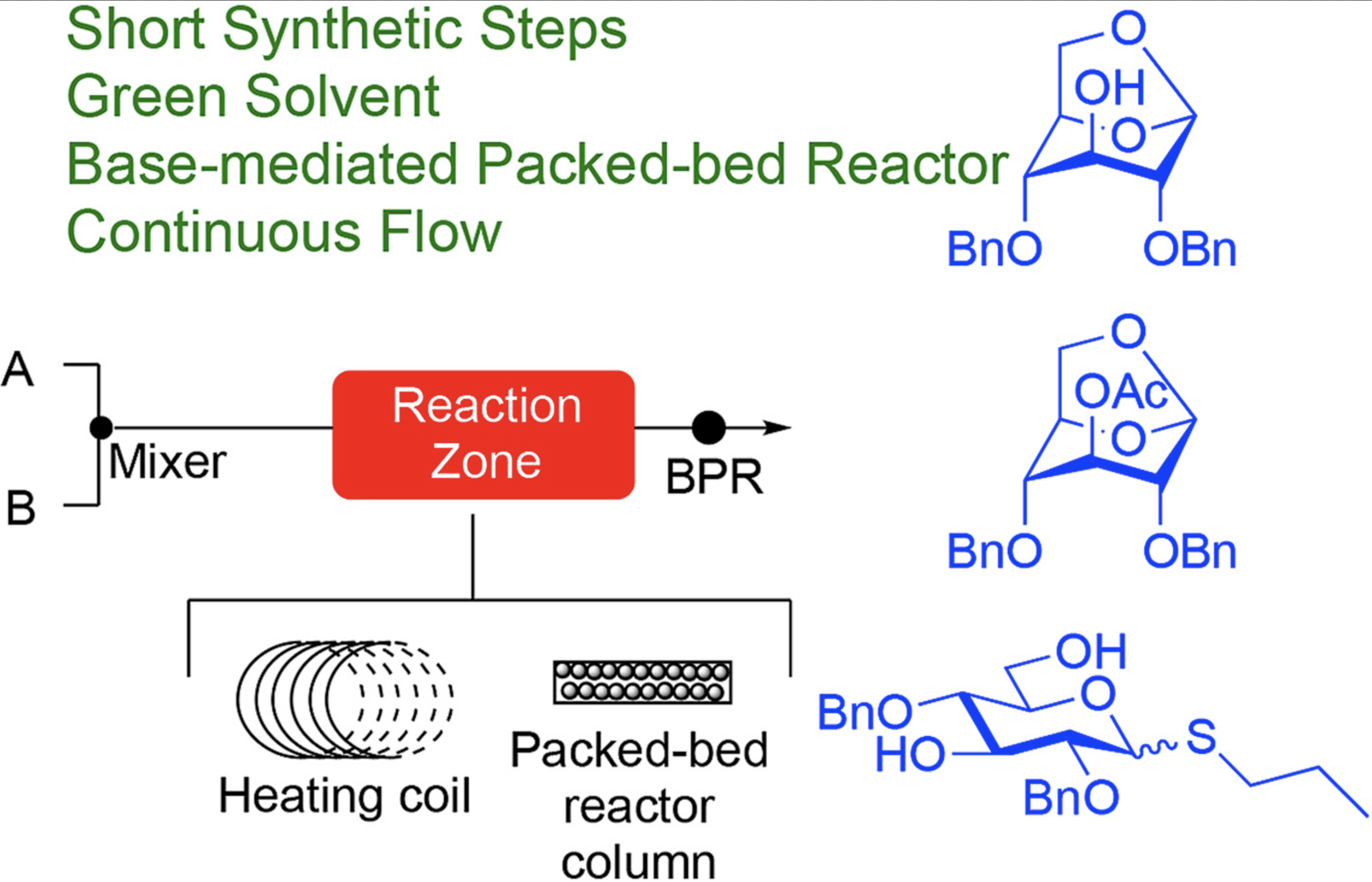
Synthesis of protected glucose derivatives from levoglucosan by development of common carbohydrate protecting group reactions under continuous flow conditions
Marion, K. C; Wooke, Z.; Pohl, N. L. B.* Synthesis of protected glucose derivatives from levoglucosan by development of common carbohydrate protecting group reactions under continuous flow conditions. Carbohydr. Res. 2018, 469, 23. DOI: /10.1016/j.carres.2018.08.002
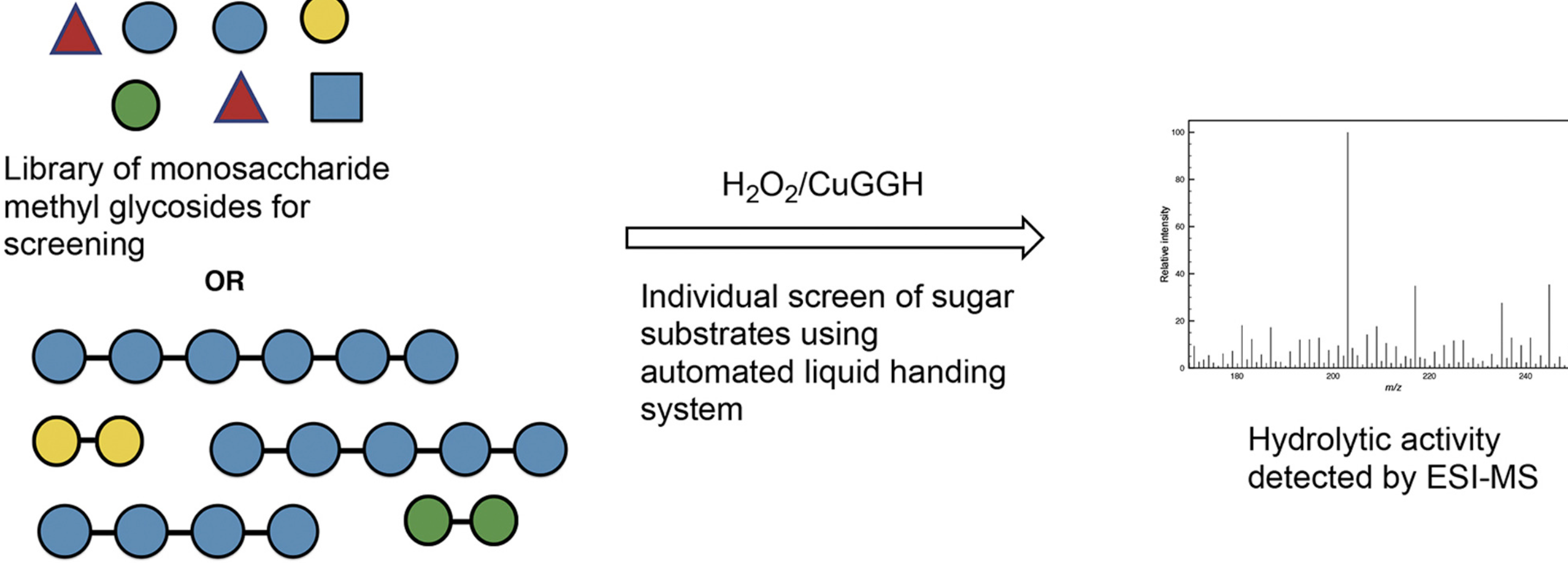
Scope and limitations of carbohydrate hydrolysis for de novo glycan sequencing using a hydrogen peroxide/metallopeptide-based glycosidase mimic
Peng, T.; Wooke, Z.; Pohl, N. L. B.* Scope and limitations of carbohydrate hydrolysis for de novo glycan sequencing using a hydrogen peroxide/metallopeptide-based glycosidase mimic. Carbohydr. Res. 2018, 458/9, 85. DOI: 10.1016/j.carres.2018.01.008
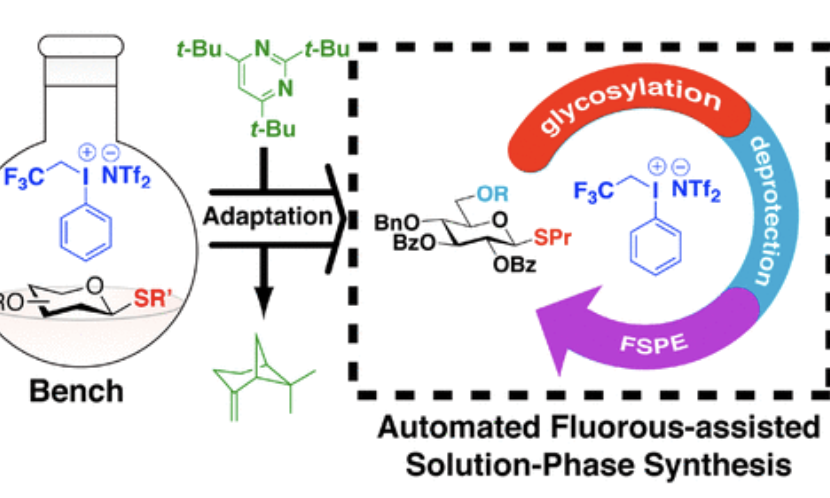
Challenges in the conversation of manual processes to machine-assisted syntheses: Activation of thioglycosides donors with aryl(trifluoroethyl)iodonium triflimide
Saliba, R. C.; Wooke, Z. J.; Nieves, G. A.; Chu A.-H. A.; Bennett, C. S.*; Pohl, N. L. B.* (*joint corresponding authors) Challenges in the conversation of manual processes to machine-assisted syntheses: Activation of thioglycosides donors with aryl(trifluoroethyl)iodonium triflimide. Org. Lett. 2018, 20, 800. DOI: 10.1021/acs.orglett.7b03940
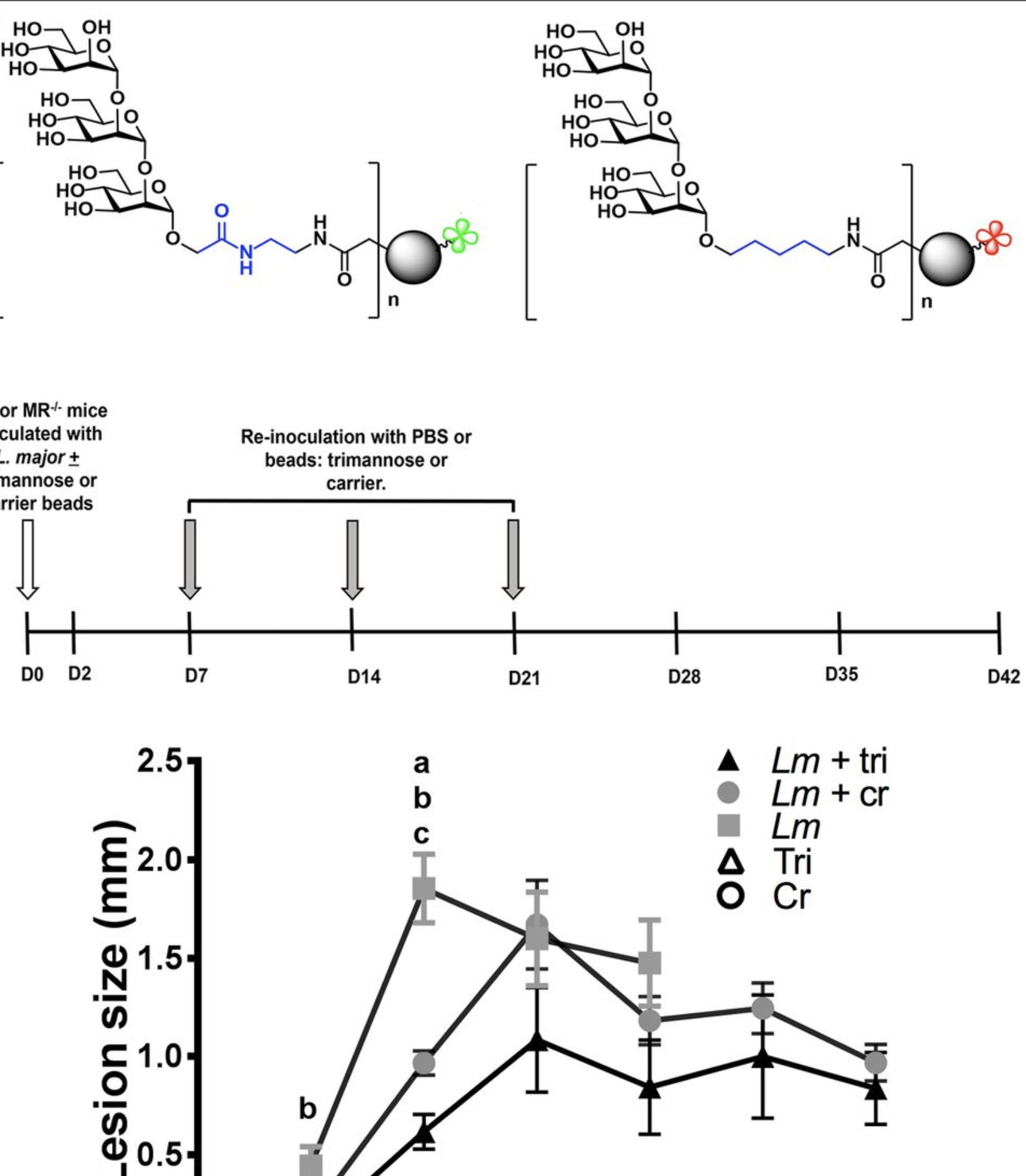
Leishmania-derived trimannose modulates inflammatory response to significantly reduce Leishmania major-induced lesions
Grinnage-Pulley, T. L.*; Kabotso, D. E. K.; Rintelmann, C. L.; Roychoudhury, R.; Schaut, R. G.; Toepp, A. J.; Gibson-Corley, K. N.; Parrish, M.; Pohl, N. L. B.; Petersen, C. A. Leishmania-derived trimannose modulates inflammatory response to significantly reduce Leishmania major-induced lesions. Infect. Immun. 2018, 86, e00672-17. DOI: 10.1128/iai.00672-17
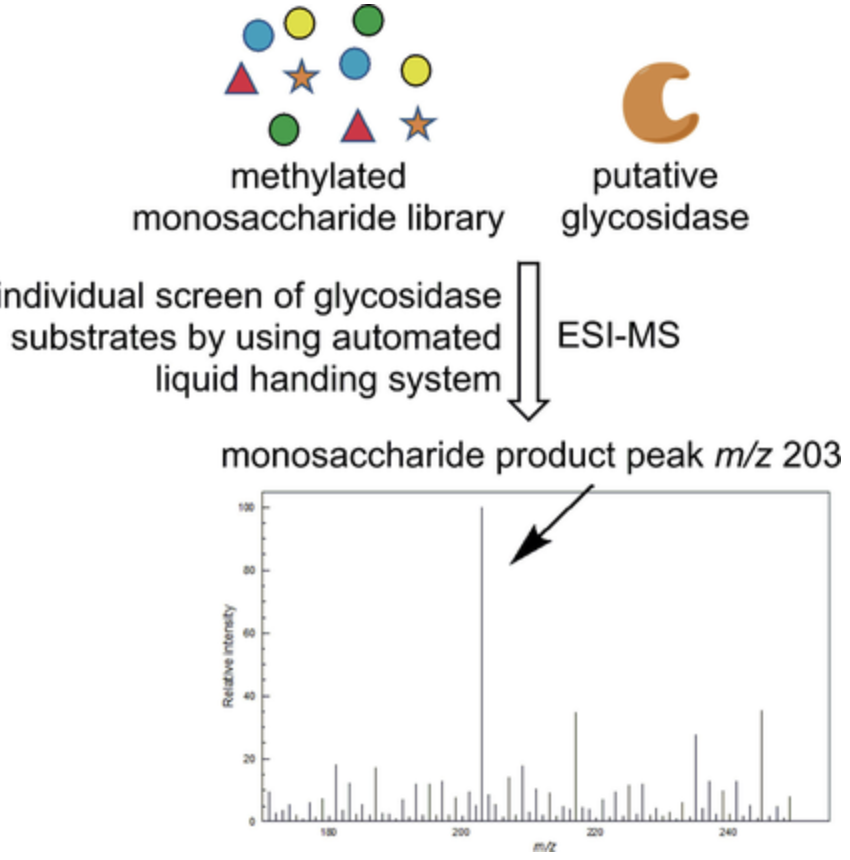
A high-throughput mass-spectrometry-based assay for identifying the biochemical functions of putative glycosidases
Peng, T.; Gabe, N.; Trinidad, J. C.; Jackson, J. M.; Pohl, N. L. B.* A high-throughput mass-spectrometry-based assay for identifying the biochemical functions of putative glycosidases. ChemBioChem, 2017, 18, 2306. DOI: 10.1002/cbic.201700292
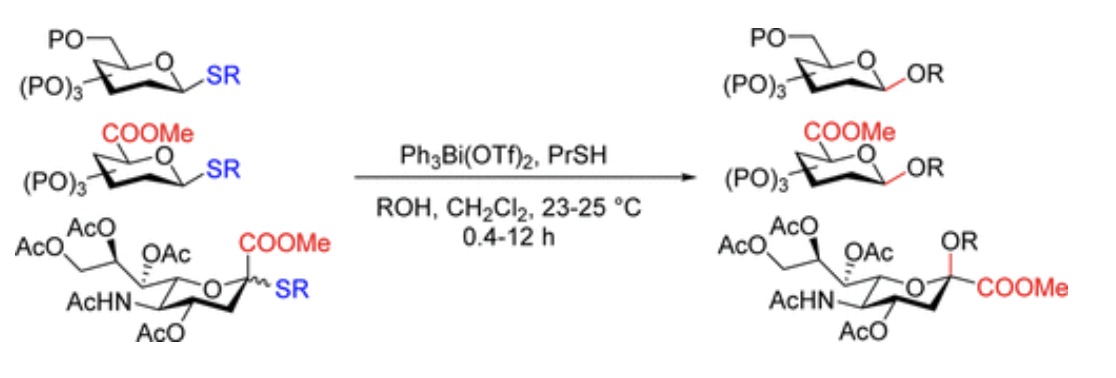
Pentavalent bismuth as a universal promoter for S-containing glycosyl donors with a thiol additive
Kabotso, D. E. K.; Pohl, N. L. B.* Pentavalent bismuth as a universal promoter for S-containing glycosyl donors with a thiol additive. Org. Lett. 2017, 19, 4516. DOI: 10.1021/acs.orglett.7b02080
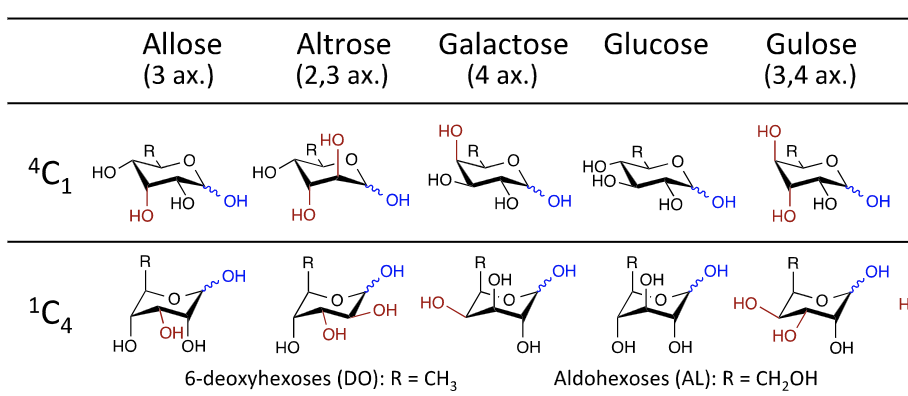
Effects of varying the 6-position oxidation state of hexopyranoses: a systematic comparative computational analysis of 48 monosaccharide stereoisomers
Vickman, A.; Ashley, D.; Baik, M.-H.*; Pohl, N. L. B.* (*joint corresponding authors) Effects of varying the 6-position oxidation state of hexopyranoses: a systematic comparative computational analysis of 48 monosaccharide stereoisomers. J. Mol. Model. 2017, 23, 214. DOI: 10.1007/s00894-017-3385-x
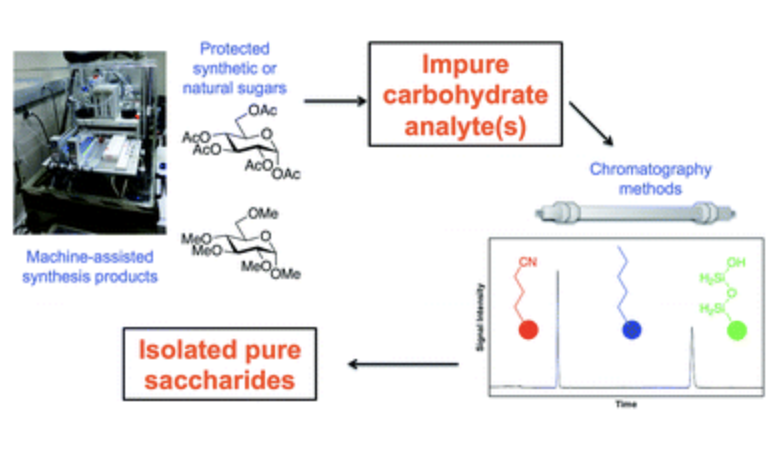
Recent liquid chromatographic approaches and developments for the separation and purification of carbohydrates
Nagy, G.‡; Peng, T.‡; Pohl, N. L. B.* (‡joint first authors) Recent liquid chromatographic approaches and developments for the separation and purification of carbohydrates. Anal. Methods 2017, 9, 3579-3593.DOI: 10.1039/C7AY01094J
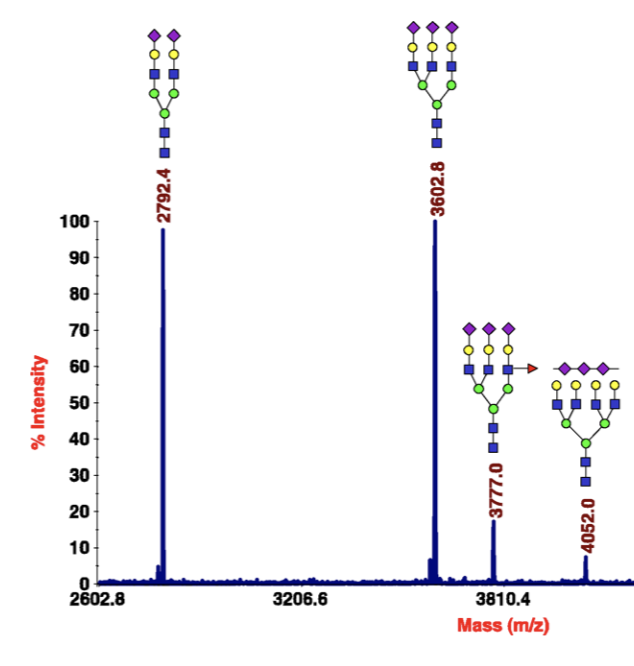
Recent advances in the analysis of complex glycoproteins
Gaunitz, S.; Nagy, G.; Pohl, N. L. B.; Novotny, M. V.* Recent advances in the analysis of complex glycoproteins. Anal. Chem. 2017, 89, 389-413. DOI: 10.1021/acs.analchem.6b04343
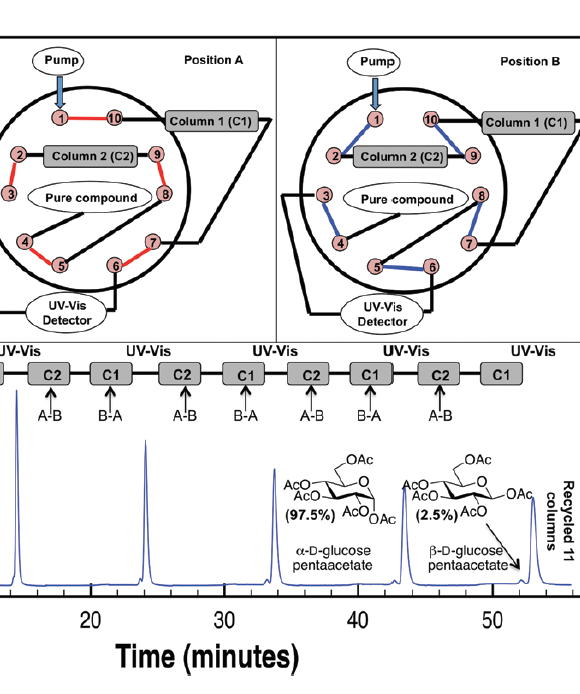
Protocol for the Purification of Protected Carbohydrates: Toward Coupling Automated Synthesis to Alternate-pump Recycling High-performance Liquid Chromatography
Nagy, G.‡; Peng, T.‡; Kabotso, D. E. K.; Novotny, M. V.; Pohl, N. L. B.* (‡joint first authors) Protocol for the Purification of Protected Carbohydrates: Toward Coupling Automated Synthesis to Alternate-pump Recycling High-performance Liquid Chromatography. Chem. Commun. 2016, 52, 13253-13256. DOI:10.1039/C6CC07584C
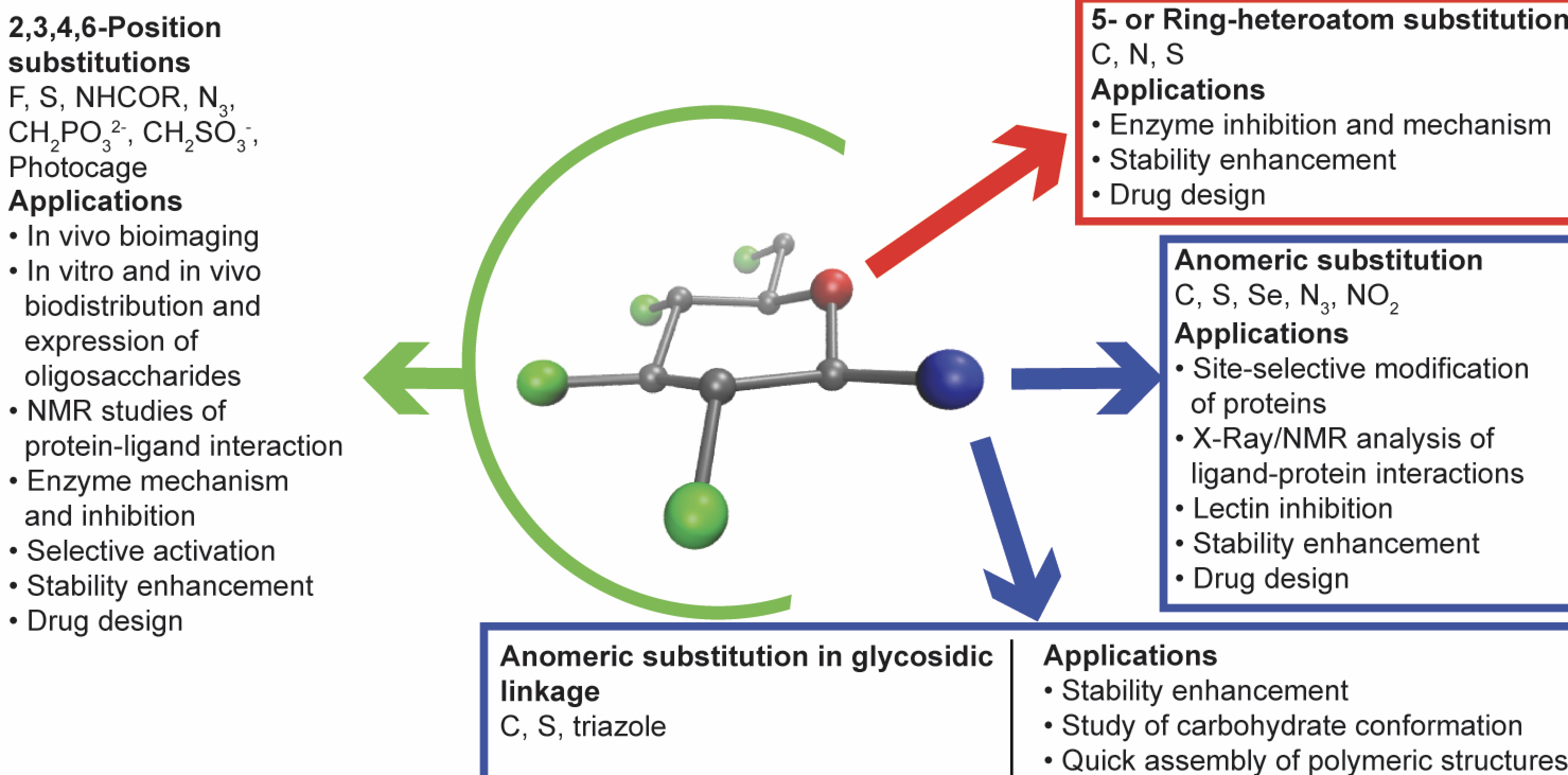
Designing Sugar Mimetics: Non-natural Pyranosides as Innovative Chemical Tools
Saliba, R. C.*; Pohl, N. L. B.* (*joint corresponding authors) Designing Sugar Mimetics: Non-natural Pyranosides as Innovative Chemical Tools. Curr. Opin. Chem. Biol. 2016, 34, 127-134. (invited review) DOI: 10.1016/j.cbpa.2016.08.027
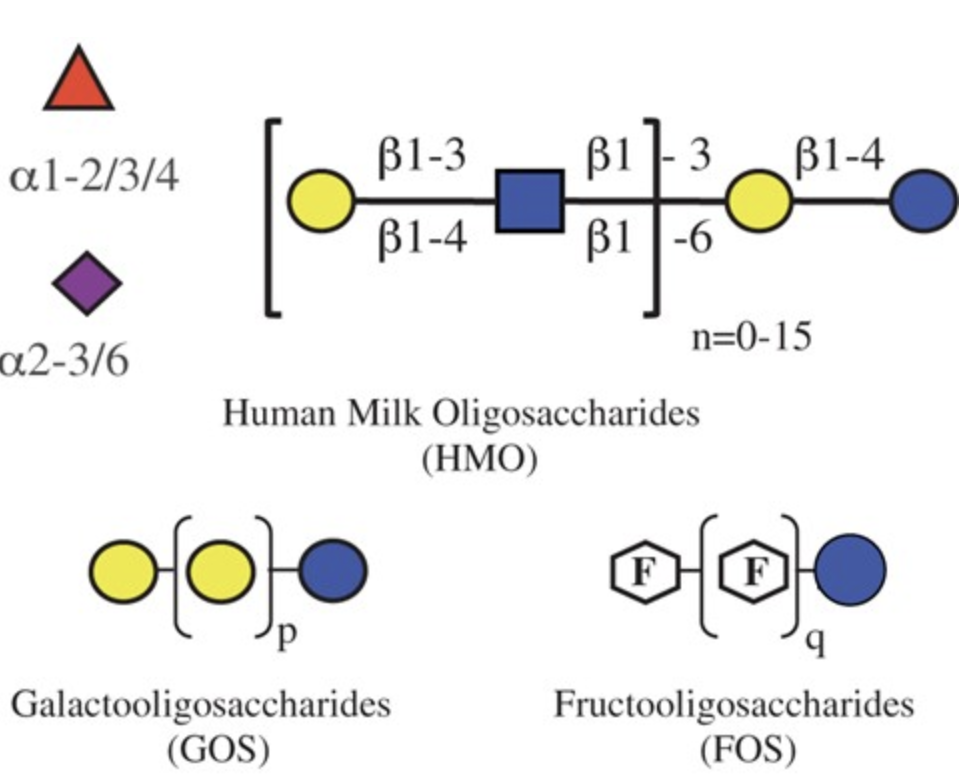
Overcoming the Limited Availability of Human Milk Oligosaccharides: Challenges and Opportunitites for Research and Application
Bode, L.*, Contractor, N.; Barile, D.; Pohl, N.; Prudden A. R., Boons, G.-J.; Jennewein, S. Overcoming the Limited Availability of Human Milk Oligosaccharides: Challenges and Opportunitites for Research and Application. Nutr. Rev. 2016, 74, 635-644. (review)DOI: 10.1093/nutrit/nuw025

General Label-Free Mass Spectrometry-Based Assay To Identify Glycosidase Substrate Competence
Nagy, G.‡; Peng, T.‡; Pohl, N. L. B.* (‡joint first authors) General Label-Free Mass Spectrometry-Based Assay To Identify Glycosidase Substrate Competence. Anal. Chem. 2016, 88, 7183-7190.

Mechanistic Studies of Bismuth(V)-Mediated Thioglycoside Activation Reveal Differential Reactivity of Anomers
Goswami, M.; Ashley, D.; Baik, M.-H.*; Pohl, N. L. B.* (*joint corresponding authors) Mechanistic Studies of Bismuth(V)-Mediated Thioglycoside Activation Reveal Differential Reactivity of Anomers. J. Org. Chem. 2016, 81, 5949-5962.

Automated Fluorous-Assisted Solution-Phase Synthesis of β-1,2-, 1,3-, and 1,6-Mannan Oligomers
Tang, S.-L.; Pohl, N. L. B.* Automated Fluorous-Assisted Solution-Phase Synthesis of β-1,2-, 1,3-, and 1,6-Mannan Oligomers. Carbohydr. Res. 2016, 430, 8-15.

Fluorous-Tag Assisted Syntheses of Sulfated Keratan Sulfate Oligosaccharide Fragments
Bhaduri, S.; Pohl, N. L. B.* Fluorous-Tag Assisted Syntheses of Sulfated Keratan Sulfate Oligosaccharide Fragments. Org. Lett. 2016, 18, 1414-1417.

Multidimensional Analysis of 16 Glucose Isomers by Ion Mobility Spectrometry
Gaye, M.*; Nagy, G.; Clemmer, D. E.; Pohl, N. L. B. Multidimensional Analysis of 16 Glucose Isomers by Ion Mobility Spectrometry. Anal. Chem. 2016, 88, 2335-2344.

Introducing Students to Protein Analysis Techniques: Separation and Comparative Analysis of Gluten Proteins in Various Wheat Strains
Pirinelli, A. L.; Trinidad, J.; Pohl, N. L. B.* Introducing Students to Protein Analysis Techniques: Separation and Comparative Analysis of Gluten Proteins in Various Wheat Strains. J. Chem. Educ. 2016, 93, 330-334.
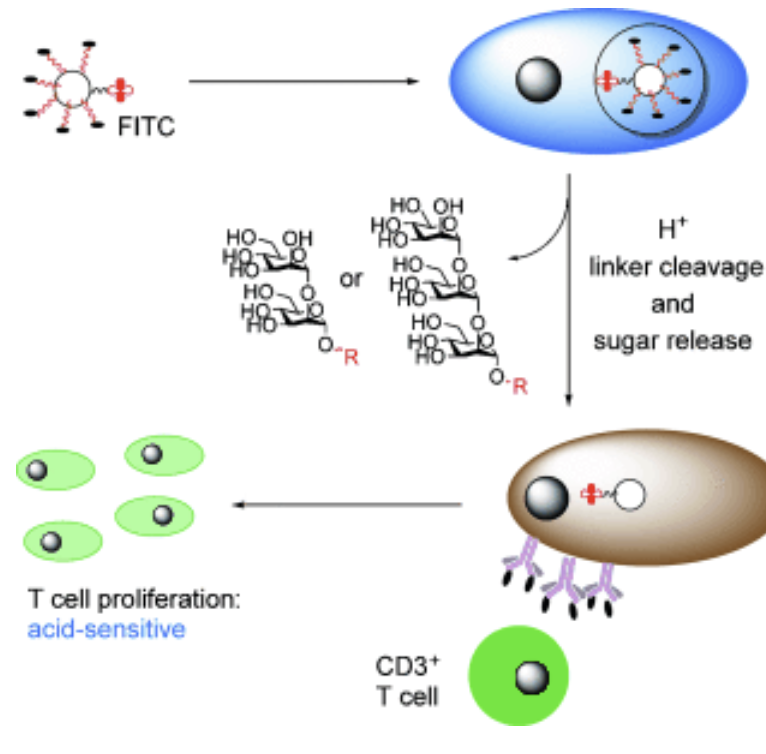
Acid-triggered degradable reagents for differentiation of adaptive and innate immune responses to Leishmania-associated carbohydrate
Roychoudhury, R.; Martinez, P.; Grinnage-Pulley, T.; Schaut, R. G.; Petersen, C. A.; Pohl, N. L. B.* Acid-triggered degradable reagents for differentiation of adaptive and innate immune responses to Leishmania-associated carbohydrate. Angew. Chem. Int. Ed. 2015, 54, 9610-9613. DOI: 10.1002/anie.201502807
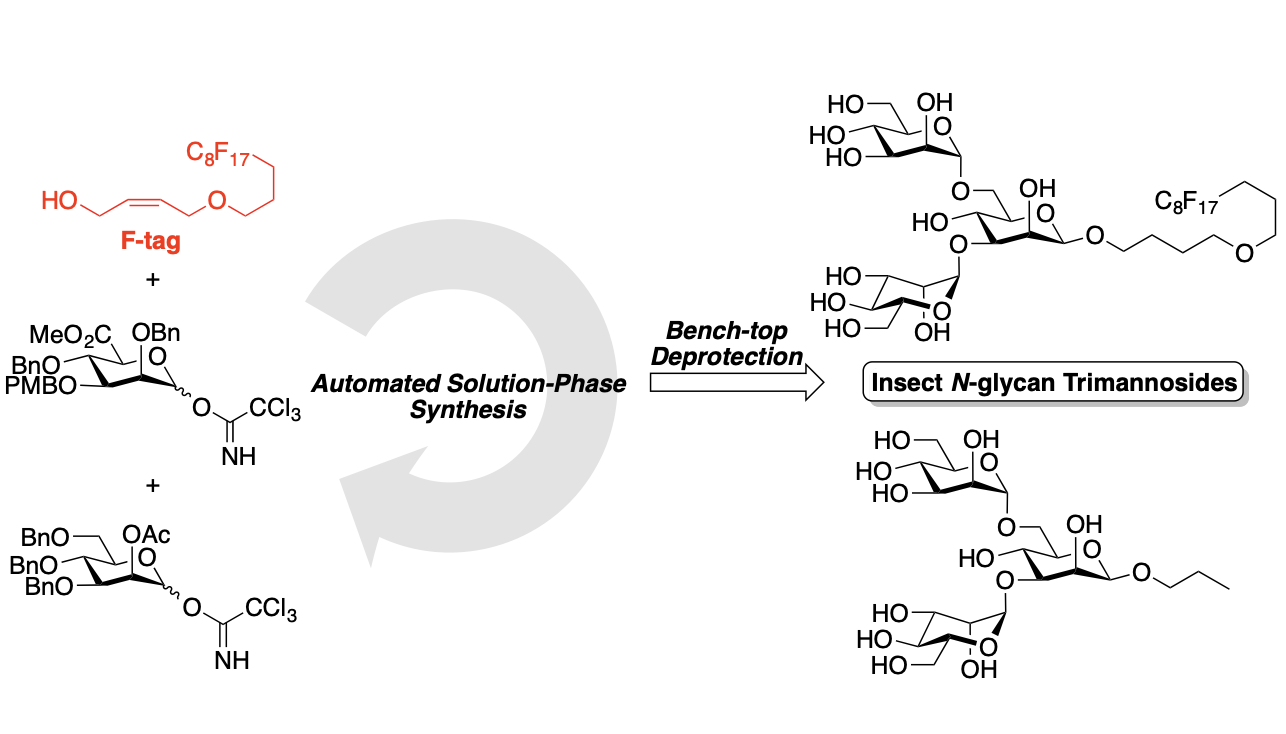
Automated Solution-Phase Synthesis of Insect Glycans to Probe the Binding Affinity of Pea Enation Mosaic Virus
Tang, S.-L.; Linz, L.; Bonning, B.; Pohl, N. L. B.* Automated Solution-Phase Synthesis of Insect Glycans to Probe the Binding Affinity of Pea Enation Mosaic Virus. J. Org. Chem. 2015, 80, 10482-10489. DOI: 10.1021/acs.joc.5b01428

Automated Solution-Phase Synthesis of β-1,4-Mannuronate and β-1,4-Mannan
Tang, S.-L.; Pohl, N. L. B.* Automated Solution-Phase Synthesis of β-1,4-Mannuronate and β-1,4-Mannan. Org. Lett. 2015, 17, 2642-2645.

Monosaccharide identification as a first step toward de novo carbohydrate sequencing: Mass spectrometry strategy for the identification and differentiation of diastereomeric and enantiomeric pentose isomers
Nagy, G.; Pohl, N. L. B.* Monosaccharide identification as a first step toward de novo carbohydrate sequencing: Mass spectrometry strategy for the identification and differentiation of diastereomeric and enantiomeric pentose isomers. Anal. Chem. 2015, 87, 677-685.
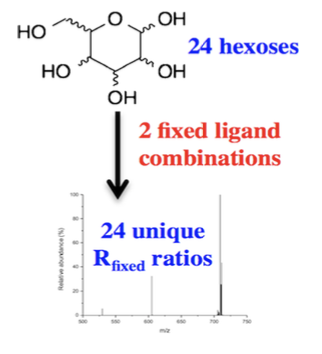
Complete Hexose Isomer Identification with Mass Spectrometry
Nagy, G.; Pohl, N. L. B.* Complete Hexose Isomer Identification with Mass Spectrometry. J. Amer. Soc. Mass Spectrom. 2015, 26, 677-685.DOI: 10.1007/s13361-014-1072-z

Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles
Vela Ramirez, J. E.; Goodman, J. T.; Boggiatto, P. M.; Roychoudhury, R.; Pohl, N. L. B.; Hostetter, J. M.; Wannemuehler, M. J.; Narasimhan, B.* Safety and biocompatibility of carbohydrate-functionalized polyanhydride nanoparticles. The AAPS Journal, 2015, 17, 256-267.

Facile access to (1→3)-glucosamine linkages: Synthesis of Propyl 4,6-di-O-Benzyl-2-Deoxy-2-N-Trichloroacetyl-1-Thio-β-D-Glucopyranoside
Mukherjee, C.; Shiao, T. C.; Pohl, N. L. B.* Facile access to (1→3)-glucosamine linkages: Synthesis of Propyl 4,6-di-O-Benzyl-2-Deoxy-2-N-Trichloroacetyl-1-Thio-β-D-Glucopyranoside. Carbohydrate Chemistry: Proven Methods, Volume 3; 2015; Roy, R.; Vidal, S., Eds.; CRC Press, ISBN-10: 1466583576.

Nanoparticle chemistry and functionalization differentially regulates dendritic cell-nanoparticle interactions and triggers dendritic cell maturation
Goodman, J. T.; Vela Ramirez, J. E.; Boggiatto, P. M.; Roychoudhury, R.; Pohl, N. L. B.; Wannemuehler, M. J.; Narasimhan, B.* Nanoparticle chemistry and functionalization differentially regulates dendritic cell-nanoparticle interactions and triggers dendritic cell maturation. Part. Part. Sys. Charact., 2014, 31, 1269-1280.

Regioselective Benzylation of 2-Deoxy-2-Aminosugars Using Crown Ethers: Application to a Shortened Synthesis of Hyaluronic Acid Oligomers
Mukherjee, C.; Lin, L.; Pohl, N. L. B.* Regioselective Benzylation of 2-Deoxy-2-Aminosugars Using Crown Ethers: Application to a Shortened Synthesis of Hyaluronic Acid Oligomers. Adv. Synth. Catal. 2014, 356, 2247-2256.

Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells
Vela Ramirez, J. E.; Roychoudhury, R.; Habte, H.; Cho, M. W.; Pohl, N. L. B.; Narasimhan, B.* Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells. J. Biomater. Sci. Polym. Ed. 2014, 25, 1387-1406.

Synthesis and Functionalization of Virus-Mimicking Cationic Block Copolymers with Pathogen-Associated Carbohydrates as Potential Vaccine Adjuvants
Adams, J.°; Goswami, M.°; Pohl N.; Mallapragada, S. K.* Synthesis and Functionalization of Virus-Mimicking Cationic Block Copolymers with Pathogen-Associated Carbohydrates as Potential Vaccine Adjuvants. RSC Advances, 2014, 4, 15655-15663. (°These two authors contributed equally to the work.)

Synthesis of Fluorous Photolabile Aldehyde and Carbamate and Alkyl Carbamate Protecting Groups for Carbohydrate-Associated Amines
Roychoudhury, R.; Pohl, N. L. B.* Synthesis of Fluorous Photolabile Aldehyde and Carbamate and Alkyl Carbamate Protecting Groups for Carbohydrate-Associated Amines. Org. Lett. 2014, 16, 1156-1159.

A Research Module for the Organic Chemistry Laboratory: Multistep Synthesis of a Fluorous Dye Molecule
Slade, M. C.*, Raker, J.; Kobilka, B.; Pohl, N. L. B.* A Research Module for the Organic Chemistry Laboratory: Multistep Synthesis of a Fluorous Dye Molecule. J. Chem. Educ. 2014, 91, 126-130.

Bismuth(V)-mediated thioglycoside activation
Goswami, M.; Ellern, A.; Pohl, N. L. B.* Bismuth(V)-mediated thioglycoside activation. Angew. Chem. Int. Ed. 2013, 52, 8441-8445.

The development of N-aryl trifluoroacetimidate-based benzyl and allyl protecting group reagents
Tsabedze, S. B.; Kabotso, D. E. K.; Pohl, N. L. B.* The development of N-aryl trifluoroacetimidate-based benzyl and allyl protecting group reagents. Tetrahedron Lett. 2013, 54, 6983-6985.
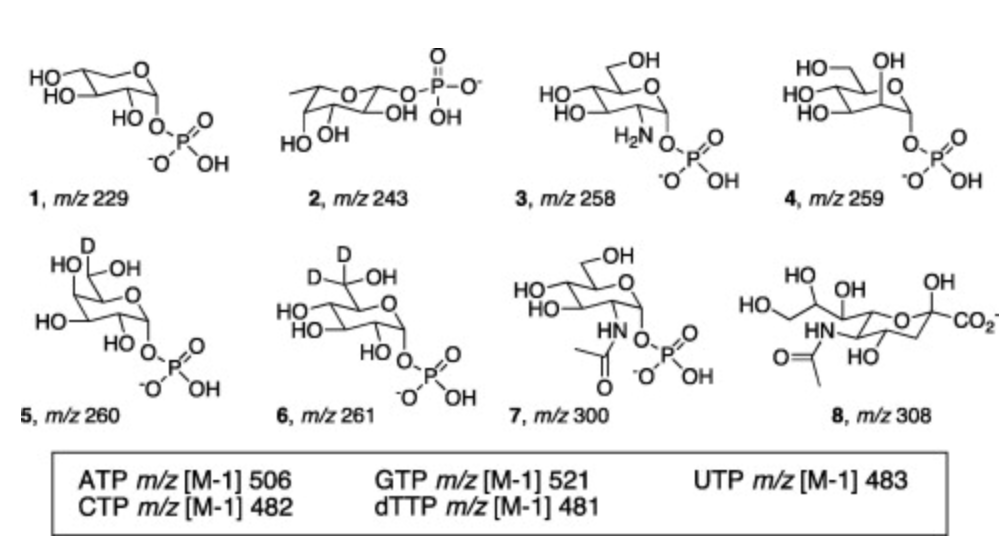
A mass-differentiated library strategy for identification of sugar nucleotidyltransferase activities from cell lysates
Ko, K.-S.; Mizanur, R. M.; Jackson, J. M.; Lin, L.; Pohl, N. L. B.* A mass-differentiated library strategy for identification of sugar nucleotidyltransferase activities from cell lysates. Anal. Biochem. 2013, 441, 8-12.DOI: 10.1016/j.ab.2013.06.004

Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells
Phanse, Y.; Carrillo-Conde, B. R.; Ramer-Tait, A. E.; Roychoudhury, R.; Pohl, N. L. B.; Narasimhan, B.*; Wannemuehler, M. J.*; Bellaire, B. H.* Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomaterialia, 2013, 9, 8902-8909.
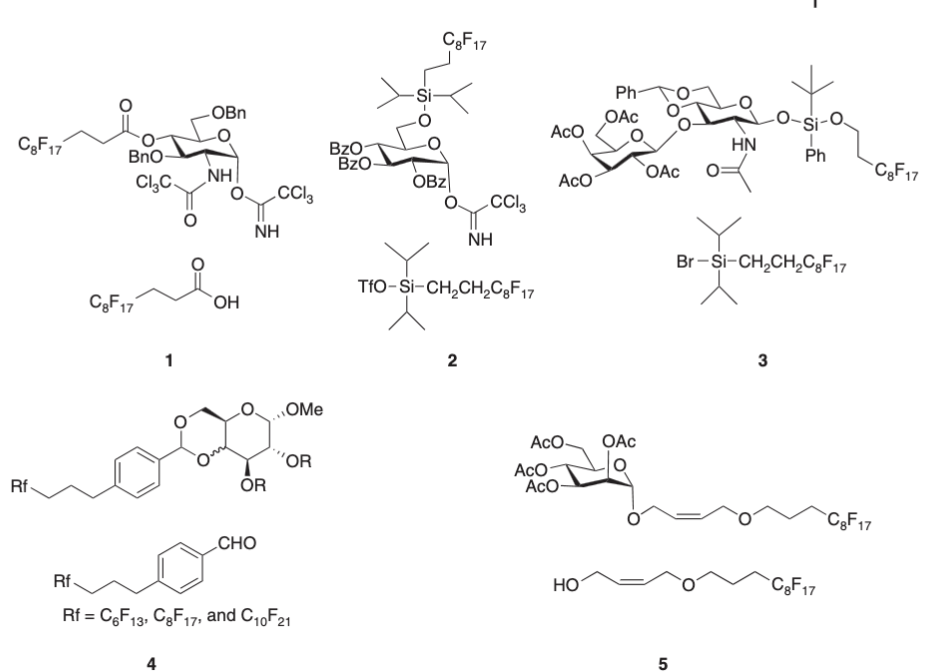
Light fluorous-tag assisted synthesis of oligosaccharides
Roychoudhury, R.; Pohl, N. L. B.* Light fluorous-tag assisted synthesis of oligosaccharides. In Modern Synthetic Methods in Carbohydrate Chemistry—From Monosaccharides to Complex Glycoconjugates; Werz, D. B.; Vidal, S. Eds. Wiley-VCH, 2013, pp. 221-240, ISBN-10: 3527332847. DOI: 10.1002/9783527658947.ch8

Synthesis of a series of maltotriose phosphates with an evaluation of the utility of a fluorous phosphate protecting group
Lin, L.; Pohl, N. L. B.* Synthesis of a series of maltotriose phosphates with an evaluation of the utility of a fluorous phosphate protecting group. Carbohydr. Res. 2013, 369, 14-24.

Substrate Binding by the Catalytic Domain and Carbohydrate Binding Module of Ruminococcus flavefaciens FD-1 Xyloglucanase/Endoglucanase
Warner, C.; Camci-Unal, G.; Pohl, N. L. B.; Reilly, P.* Substrate Binding by the Catalytic Domain and Carbohydrate Binding Module of Ruminococcus flavefaciens FD-1 Xyloglucanase/Endoglucanase. Ind. Eng. Chem. Res. 2013, 52, 30-36. (special issue)

Synthesis of a 3-deoxy-D-manno-octulosonic acid (KDO) building block from D-glucose via fermentation
Camci-Unal, G.; Mizanur, R. M.; Pohl, N. L. B.* Synthesis of a 3-deoxy-D-manno-octulosonic acid (KDO) building block from D-glucose via fermentation. Org. Biomol. Chem. 2012, 10, 5856-5860. (special issue)
High-throughput synthesis of carbohydrates and functionalization of polyanhydride nanoparticles
Carillo-Conde, B. R.; Roychoudhury, R.; Chaves-Santoscoy, A. V.; Narasimhan, B.; Pohl, N. L. High-throughput synthesis of carbohydrates and functionalization of polyanhydride nanoparticles. J. Vis. Exp. 2012, 65, e3967. DOI: 10.3791/3967-v
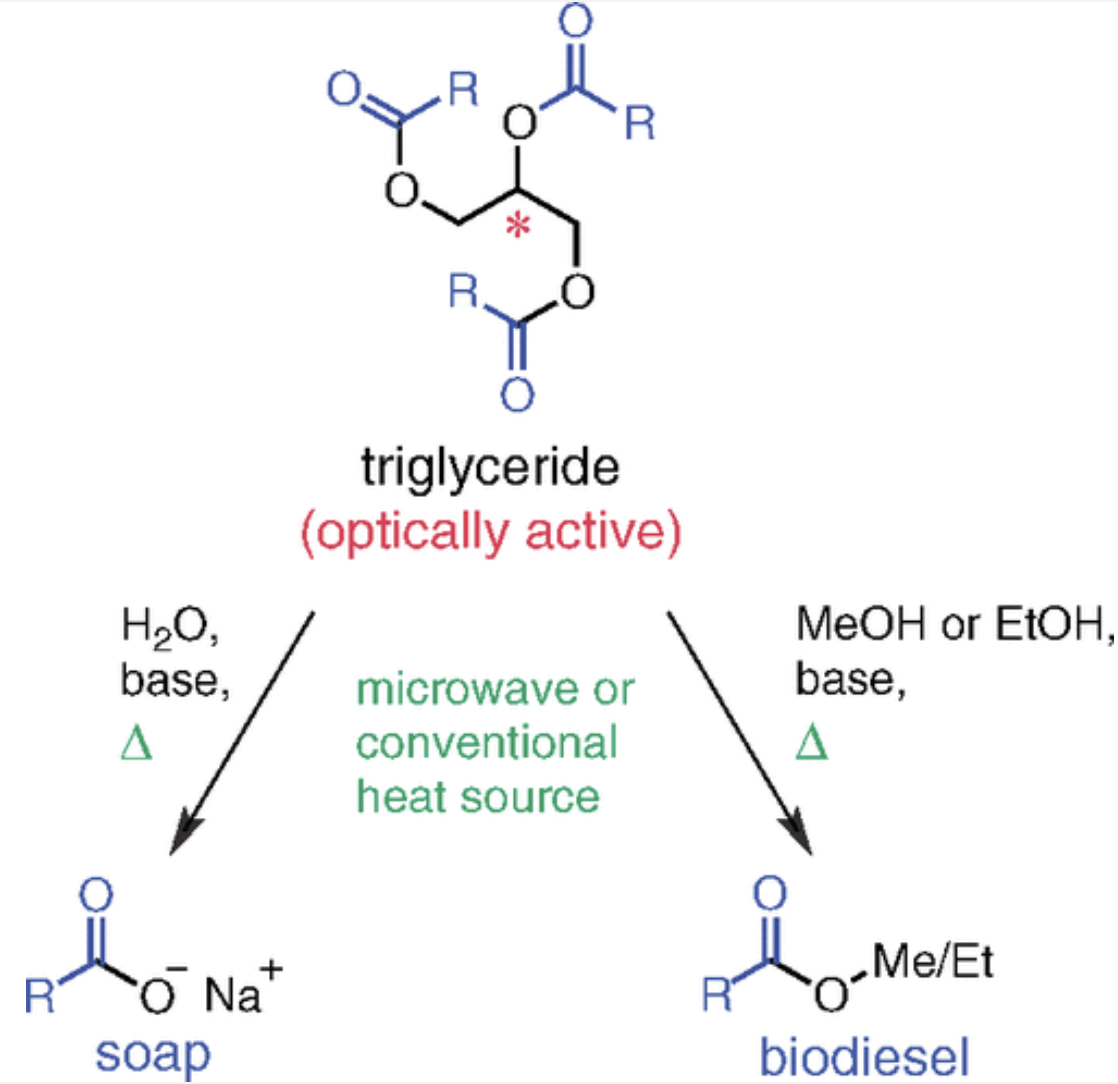
Evaluating Sustainability: Soap versus Biodiesel Production from Plant Oils
Pohl, N. L. B.*; Streff, J.; Brokman, S. Evaluating Sustainability: Soap versus Biodiesel Production from Plant Oils. J. Chem. Educ. 2012, 89, 1053-1056. DOI: 10.1021/ed100451d

Tailoring the Immune Response by Targeting C-type Lectin Receptors on Alveolar Macrophages Using “Pathogen-like” Amphiphilic Polyanhydride Nanoparticles”
Chavez-Santoscoy, A.; Roychoudhury, R.; Pohl, N. L. B.; Wannemuehler, M. J.; Narasimhan, B.*; Ramer-Tait, A. E. Tailoring the Immune Response by Targeting C-type Lectin Receptors on Alveolar Macrophages Using “Pathogen-like” Amphiphilic Polyanhydride Nanoparticles.” Biomaterials, 2012, 33, 4762-4772.

Multigram Synthesis of Isobutyl-beta-C-galactoside as a Substitute of Isopropylthiogalactoside for Exogenous Gene Induction in Mammalian Cells
Lin, L.; Abdel Motaal, B.; Schmidt-Supprian, M.; Pohl, N. L. B.* Multigram Synthesis of Isobutyl-beta-C-galactoside as a Substitute of Isopropylthiogalactoside for Exogenous Gene Induction in Mammalian Cells. J. Org. Chem. 2012, 77, 1539-1546.

Probing the Limitations of Fluorous Content for Tag-Mediated Microarray Formation
Edwards, H. E.; Nagappayya, S. K.; Pohl, N. L. B.* Probing the Limitations of Fluorous Content for Tag-Mediated Microarray Formation. Chem. Commun. 2012, 48, 510-512.

Production of Fluorous-Based Microarrays with Uncharged Carbohydrates
Nagappayya, S. K.; Pohl, N. L. B.* Production of Fluorous-Based Microarrays with Uncharged Carbohydrates. In Carbohydrate Microarrays: Methods and Protocols. Methods in Molecular Biology, vol. 808, Chevolot, Y., Ed. Springer, 2011, 149-153. (invited book chapter)

Mannose-Functionalized “Pathogen-Like” Polyanhydride Nanoparticles Target C-Type Lectin Receptors on Dendritic Cells
Carrillo-Conde, B.; Song, E.-H.; Chavez-Santoscoy, A.; Phanse, Y.; Ramer-Tait, A. E.; Pohl, N. L. B.; Wannemuehler, M. J.; Bellaire, B. H.; Narasimhan, B.* Mannose-Functionalized “Pathogen-Like” Polyanhydride Nanoparticles Target C-Type Lectin Receptors on Dendritic Cells. Mol. Pharmaceutics, 2011, 8, 1877-1886.

Pathogen-derived oligosaccharides improve innate immune response to intracellular parasite infection
Osanya, A.; Song, E.-H.; Metz, K.; Shimak, R. M.; Boggiatto, P. M.; Huffman, E.; Johnson, C.; Hostetter, J. M.; Pohl, N. L. B.; Petersen, C. A.* Pathogen-derived oligosaccharides improve innate immune response to intracellular parasite infection. Am. J. Pathol. 2011, 179, 1329-1337.

Student-driven design of peptide mimetics: microwave-assisted synthesis of peptoid oligomers
Pohl, N. L. B.*; Kirshenbaum, K.; Yoo, B.; Schulz, N.; Zea, C. J.; Streff, J. M.; Schwarz, K. L. Student-driven design of peptide mimetics: microwave-assisted synthesis of peptoid oligomers. J. Chem. Educ. 2011, 88, 999-1001.

A Fluorous Phosphate Protecting Group with Applications in Carbohydrate Synthesis
Lin, L.; Pohl, N. L. B.* A Fluorous Phosphate Protecting Group with Applications in Carbohydrate Synthesis. Org. Lett. 2011, 13, 1824-1827.

Synthesis of multivalent tuberculosis and Leishmania-associated capping carbohydrates reveals structure-dependent responses allowing immune evasion
Song, E.-H.; Osanya, A. O.; Petersen, C. A.; Pohl, N. L. B.* Synthesis of multivalent tuberculosis and Leishmania-associated capping carbohydrates reveals structure-dependent responses allowing immune evasion. J. Am. Chem. Soc. 2010, 132, 11428-11430.

Spectral and thermodynamic properties of methanobactin from gamme-proteobacterial methane oxidizing bacteria: A case for copper competition on a molecular level
Choi, D. W.; Bandow, N. L.; McEllistrem, M. T.; Semrau, J. D.; Antholine, W. W.; Hartsel, S. C.; Gallagher, W.; Zea, C. J.; Pohl, N. L.; Zahn, J. A.; DiSpirito, A. A.* Spectral and thermodynamic properties of methanobactin from gamme-proteobacterial methane oxidizing bacteria: A case for copper competition on a molecular level. J. Inorg. Biochem. 2010, 104, 1240-1247.

Thermodynamics of Binding Interactions between Divalent Copper and Chitin Fragments by Isothermal Titration Calorimetry (ITC)
Camci-Unal, G.; Pohl, N. L. B.* Thermodynamics of Binding Interactions between Divalent Copper and Chitin Fragments by Isothermal Titration Calorimetry (ITC). Carbohydr. Polym. 2010, 81, 8-13.

New Structures, Chemical Functions, and Inhibitors for Glycosyltransferases
Roychoudhury, R.; Pohl, N. L. B.* New Structures, Chemical Functions, and Inhibitors for Glycosyltransferases. Curr. Opin. Chem. Biol. 2010, 14, 168-173. (invited review)

The Sugar Code: Fundamentals of Glycosciences
Pohl, N.* The Sugar Code: Fundamentals of Glycosciences. ChemBioChem, 2010, 11, 1147. (invited book review)

Quantitative Determination of Heavy Metal Contaminant Complexation by the Carbohydrate Polymer Chitin
Camci-Unal, G.; Pohl, N. L. B.* Quantitative Determination of Heavy Metal Contaminant Complexation by the Carbohydrate Polymer Chitin. J. Chem. Eng. Data, 2010, 55, 1117-1121.

Rapid multistep synthesis of a bioactive peptidomimetic oligomer for the undergraduate lab
Utku, Y.; Rohatgi, A.; Yoo, B.; Zuckermann, R. N.; Pohl, N. L.; Kirshenbaum, K.* Rapid multistep synthesis of a bioactive peptidomimetic oligomer for the undergraduate lab. J. Chem. Educ. 2010, 87, 637-639.

Carbohydrate arrays: recent developments in fabrication and detection methods with applications
Song, E.-H.; Pohl, N. L. B.* Carbohydrate arrays: recent developments in fabrication and detection methods with applications. Curr. Opin. Chem. Biol. 2009, 13, 626-632. (invited review)
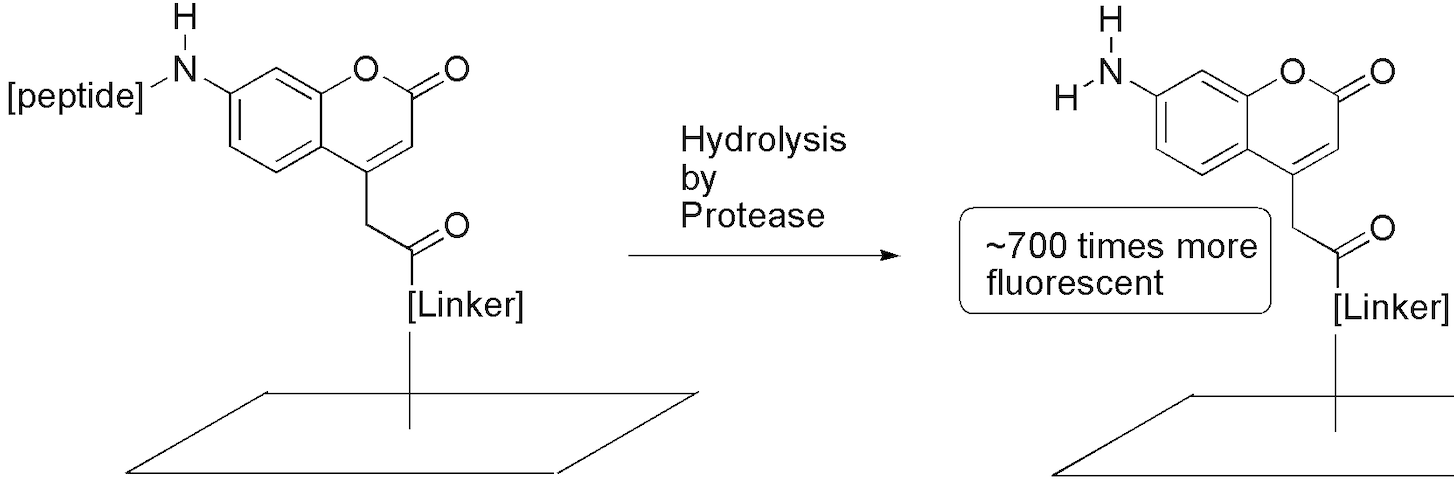
Fluorous-based Peptide Microarrays for Protease Screening
Collet, B. Y. M.; Nagashima, T.; Yu, M. S.; Pohl, N. L. B.* Fluorous-based Peptide Microarrays for Protease Screening. J. Fluorine Chem. 2009, 130, 1042-1048. DOI: 10.1016/j.jfluchem.2009.09.005

Fluorous-based small-molecule microarrays for protein, antibody, and enzyme screening
Song, E.-H.; Pohl, N. L. B.* Fluorous-based small-molecule microarrays for protein, antibody, and enzyme screening. Future Med. Chem. 2009, 1, 889-896. (invited special report)

The expanding world of glycobiology
Pohl, N. L. B. The expanding world of glycobiology. Nature Chem. Biol. 2009, 5, 373. (invited book review)

Phosphomannose isomerase/GDP-mannose pyrophosphorylase from Pyrococcus furiosus: a thermostable biocatalyst for the synthesis of guanidinediphosphate-activated and mannose-containing sugar nucleotides
Mizanur, R. M.; Pohl, N. L.* Phosphomannose isomerase/GDP-mannose pyrophosphorylase from Pyrococcus furiosus: a thermostable biocatalyst for the synthesis of guanidinediphosphate-activated and mannose-containing sugar nucleotides, Org. Biomol. Chem. 2009, 7, 2135-2139.

Protecting Group-based Colorimetric Monitoring of Fluorous-phase and Solid-phase Synthesis of Oligoglucosamines
Ko, K.-S.; Park, G.; Yu, Y.; Pohl, N. L.* Protecting Group-based Colorimetric Monitoring of Fluorous-phase and Solid-phase Synthesis of Oligoglucosamines. Org. Lett. 2008, 10, 5381-5384.

Bacterial CMP-Sialic Acid Synthetases: Production, Properties and Applications
Mizanur, R. M.*; Pohl, N. L.* Bacterial CMP-Sialic Acid Synthetases: Production, Properties and Applications. Appl. Microbiol. Biotechnol. 2008, 80, 757-765. (invited review)

Thermodynamics of Binding of Divalent Magnesium and Manganese to Uridine Phosphates: Implications for Diabetes-Related Hypomagnesaemia and Carbohydrate Biocatalysis
Zea, C. J.; Camci-Unal, G.; Pohl, N. L.* Thermodynamics of Binding of Divalent Magnesium and Manganese to Uridine Phosphates: Implications for Diabetes-Related Hypomagnesaemia and Carbohydrate Biocatalysis. Chem. Cent. J., 2008, 2, 15.

Automated Solution-Phase Oligosaccharide Synthesis and Carbohydrate Microarrays: Development of Fluorous-Based Tools for Glycomics
Pohl, N. L.* Automated Solution-Phase Oligosaccharide Synthesis and Carbohydrate Microarrays: Development of Fluorous-Based Tools for Glycomics. In Chemical Glycobiology; Chen, X.; Halcomb, R.; Wang, G. P., Eds. ACS Symposium Series 990; American Chemical Society: Washington, DC, 2008, pp. 272-287. (invited book chapter)

Toward Solution-Phase Automated Iterative Synthesis: Fluorous-tag Assisted Solution-Phase Synthesis of Linear and Branched Mannose Oligomers
Jaipuri, F. A.; Pohl, N. L.* Toward Solution-Phase Automated Iterative Synthesis: Fluorous-tag Assisted Solution-Phase Synthesis of Linear and Branched Mannose Oligomers, Org. Biomol. Chem. 2008, 6, 2686-2691.
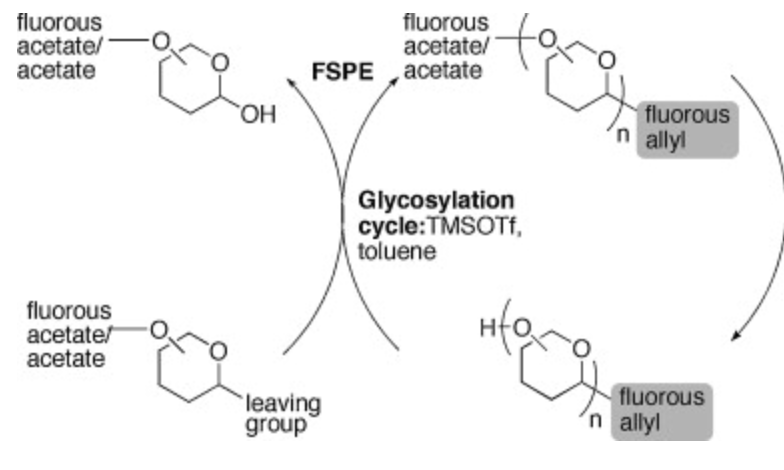
Mono- Vs. Di-fluorous Tagged Glucosamines for Iterative Oligosaccharide Synthesis
Park, G.; Ko, K.-S.; Zakharova, A.; Pohl, N. L.* Mono- Vs. Di-fluorous Tagged Glucosamines for Iterative Oligosaccharide Synthesis. J. Fluorine Chem. 2008, online. (special issue) DOI: 10.1016/j.jfluchem.2008.05.001
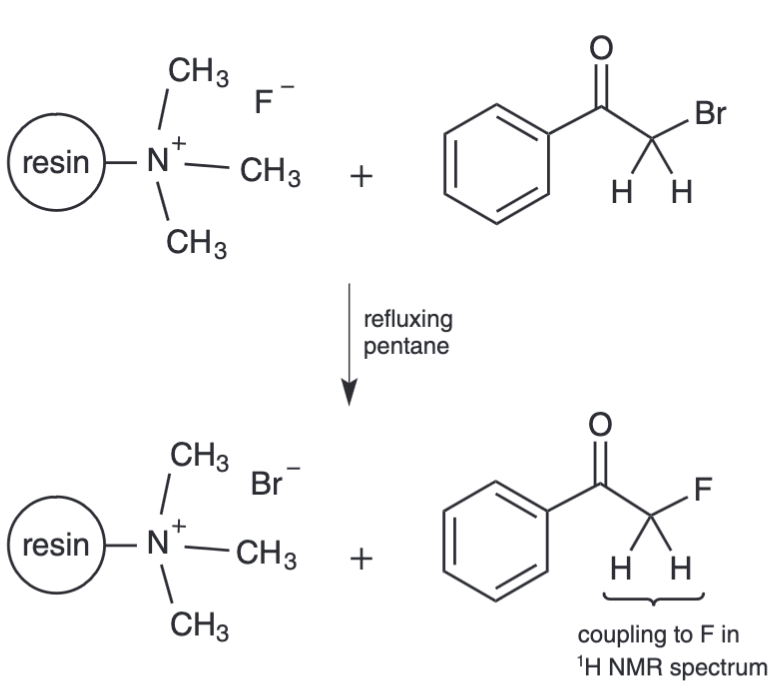
Schwarz, K. Polymer-Supported Reagents and 1H-19F NMR Couplings: The Synthesis of 2-Fluoroacetophenone
Pohl, N.*; Schwarz, K. Polymer-Supported Reagents and 1H-19F NMR Couplings: The Synthesis of 2-Fluoroacetophenone. J. Chem. Educ. 2008, 85, 834-835. DOI: 10.1021/ed085p834
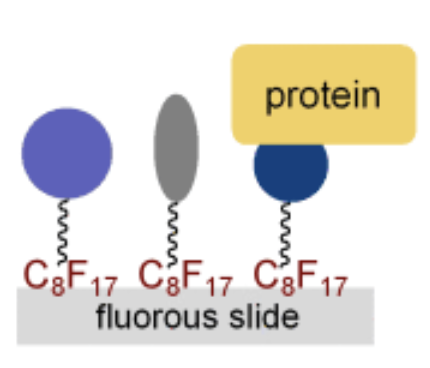
Fluorous Tags Catching on Microarrays
Pohl, N. L.* Fluorous Tags Catching on Microarrays. Angew. Chem. Int. Ed. 2008, 47, 3868-3870. (invited review) DOI: 10.1002/anie.200704801

Linkage Position and Residue Identification of Disaccharides by Tandem MS and Linear Discriminant Analysis
Hui, Z.; Brokman, S.; Fang, N.; Pohl, N.; Yeung, E.* Linkage Position and Residue Identification of Disaccharides by Tandem MS and Linear Discriminant Analysis, Rapid Commun. Mass Spectrom. 2008, 22, 1579-1586.

Synthesis and Quantitative Evaluation of Glycero-D-manno-heptose Binding to Concanavalin A by Fluorous-tag Assistance
Jaipuri, F. A.; Collet, B. Y. M.; Pohl, N. L.* Synthesis and Quantitative Evaluation of Glycero-D-manno-heptose Binding to Concanavalin A by Fluorous-tag Assistance. Angew. Chem. Int. Ed. 2008, 47, 1707-1710.

Synthesis of Fluorous Tags for Incorporation of Reducing Sugars into a Quantitative Microarray Platform
Chen, G.-S.; Pohl, N. L.* Synthesis of Fluorous Tags for Incorporation of Reducing Sugars into a Quantitative Microarray Platform. Org. Lett. 2008, 10, 785-788.

A thermostable promiscuous glucose-1-phosphate uridyltransferase from Helicobacter pylori for the synthesis of nucleotide sugars
Mizanur, R. M.; Pohl, N. L.* A thermostable promiscuous glucose-1-phosphate uridyltransferase from Helicobacter pylori for the synthesis of nucleotide sugars. J. Mol. Catal. B: Enzymatic, 2008, 50, 13-19.

Glycosidase activity profiling for bacterial identification by a chemical proteomics approach
Yu, Y.; Mizanur, R. M.; Pohl, N. L.* Glycosidase activity profiling for bacterial identification by a chemical proteomics approach. Biocatal. Biotransform. 2008, 26, 25-31.

Recombinant production and biochemical characterization of a hyperthermostable alpha-glucan/maltodextrin phosphorylase from Pyrococcus furiosus
Mizanur, R. M.; Griffin, A. K. K.; Pohl, N. L.* Recombinant production and biochemical characterization of a hyperthermostable alpha-glucan/maltodextrin phosphorylase from Pyrococcus furiosus. Archaea, 2007, 2, 169-176.

Cloning and characterization of a heat-stable CMP-N-acylneuraminic acid synthetase from Clostridium thermocellum
Mizanur, R. M.; Pohl, N. L.* Cloning and characterization of a heat-stable CMP-N-acylneuraminic acid synthetase from Clostridium thermocellum. Appl. Microbiol. Biotechnol. 2007, 76, 827-834.

Carbohydrate Microarrays and Fluorous-Phase Synthesis: Interfacing Fluorous-Phase Tags with the Direct Formation of Glycoarrays. In Current Fluoroorganic Chemistry: New Synthetic Directions, Technologies, Materials, and Biological Applications
Pohl, N. L.* Carbohydrate Microarrays and Fluorous-Phase Synthesis: Interfacing Fluorous-Phase Tags with the Direct Formation of Glycoarrays. In Current Fluoroorganic Chemistry: New Synthetic Directions, Technologies, Materials, and Biological Applications; Soloshonok, V. A.; Mikami, K.; Yamazaki, T.; Welch, J. T.; Honek, J. F., Eds. ACS Symposium Series 949; American Chemical Society: Washington, DC, 2007, pp. 261-270. (invited book chapter)

Noncovalent Fluorous Interactions for the Synthesis of Carbohydrate Microarrays
Mamidyala, S. K.; Ko, K.-S.; Jaipuri, F. A.; Park, G.; Pohl, N. L.* Noncovalent Fluorous Interactions for the Synthesis of Carbohydrate Microarrays. J. Fluorine Chem. 2006, 127, 571-579. (special issue)

Spectral and Thermodynamic Properties of Ag(I), Au(III), Cd(II), Co(II), Fe(III), Hg(II), Mn(II), Ni(II), Pb(II), U(IV), and Zn(II) Binding by Methanobactin from Methylosinus trichosporium OB3b
Choi, D. W.; Do, Y. S.; Zea, C. J.; McEllistrem, M. T.; Lee, S.-W.; Semrau, J. D.; Pohl, N. L.; Kisting, C. J.; Scardino, L. L.; Hartsel, S. C.; Boyd, E. S.; Gessey, G. G.; Riedel, T. P.; Shafe, P. H.; Kranski, K. A.; Tritsch, J. R.; Antholine, W. W.; DiSpirito, A. A.* Spectral and Thermodynamic Properties of Ag(I), Au(III), Cd(II), Co(II), Fe(III), Hg(II), Mn(II), Ni(II), Pb(II), U(IV), and Zn(II) Binding by Methanobactin from Methylosinus trichosporium OB3b. J. Inorg. Biochem. 2006, 100, 2150-2161. (special issue)

Substrate Specificity of Bacterial Oligosaccharyltransferase Suggests a Common Transfer Mechanism for the Bacterial and Eukaryotic Systems
Wacker, M.; Feldman, M. F.; Callewaert, N.; Kowarik, M.; Clarke, B. R.; Pohl, N. L.; Hernandez, M.; Vines, E. D.; Valvano, M. A.; Whitfield, C.; Aebi, M.* Substrate Specificity of Bacterial Oligosaccharyltransferase Suggests a Common Transfer Mechanism for the Bacterial and Eukaryotic Systems . Proc. Natl Acad. Sci., USA, 2006, 103, 7088-7093.

Building a Bridge to New Antibiotics
Pohl, N. L.* Building a Bridge to New Antibiotics. ACS Chem. Biol. 2006, 1, 14-16. (invited commentary)

Array methodology singles out pathogenic bacteria
Pohl, N. L.* Array methodology singles out pathogenic bacteria. Nature Chem. Biol. 2006, 2, 125-126. (invited commentary)

Spectral, Kinetic, and Thermodynamic Properties of Cu(I)- and Cu(II)-binding by Methanobactin from Methylosinus trichosporium OB3b
Choi, D. W.; Zea, C. J; Do, Y. S.; Semrau, J. D.; Antholine, W. E.; Hargrove, M. S.; Pohl, N. L.; Boyd, E. S.; Geesey, G. G.; Hartsel, S. C.; Shafe, P. H.; McEllistrem, M. T.; Kisting, C. J.; Campbell, D.; Rao, V.; de la Mora, A. M.; DiSpirito, A. A.* Spectral, Kinetic, and Thermodynamic Properties of Cu(I)- and Cu(II)-binding by Methanobactin from Methylosinus trichosporium OB3b. Biochemistry, 2006, 45, 1442-1453.

Acyclic Peptide Inhibitors of Amylases
Pohl, N.* Acyclic Peptide Inhibitors of Amylases. Chem. Biol. 2005, 12, 1257-1258. (invited commentary)

Fluorous-Based Carbohydrate Microarrays
Ko, K.-S.; Jaipuri, F. A.; Pohl, N. L.* Fluorous-Based Carbohydrate Microarrays. J. Am. Chem. Soc. 2005, 127, 13162-13163.

Unusual Sugar Nucleotide Recognition Elements of Mesophilic Versus Thermophilic Glycogen Synthases
Zea, C. J.; Pohl, N. L.* Unusual Sugar Nucleotide Recognition Elements of Mesophilic Versus Thermophilic Glycogen Synthases. Biopolymers, 2005, 79, 106-113.
![Platinum(II) Complex as an Artificial Peptidase: Selective Cleavage of Peptides and a Protein by cis-[Pt(en)(H2O)2]2+ Ion under Different Irradiations](https://pohl.lab.indiana.edu/wp-content/plugins/ezpublications//assets/images/default-pic.jpg)
Platinum(II) Complex as an Artificial Peptidase: Selective Cleavage of Peptides and a Protein by cis-[Pt(en)(H2O)2]2+ Ion under Different Irradiations
Dutca, L.-M.; Ko, K.-S.; Pohl, N. L.; Kostic, N. M.* Platinum(II) Complex as an Artificial Peptidase: Selective Cleavage of Peptides and a Protein by cis-[Pt(en)(H2O)2]2+ Ion under Different Irradiations. Inorg. Chem. 2005, 44, 5141-5146.

Strategies for the Chemoenzymatic Synthesis of Deoxysugar Nucleotides: Substrate Binding Versus Catalysis
Ko, K.-S.; Zea, C. J.; Pohl, N. L.* Strategies for the Chemoenzymatic Synthesis of Deoxysugar Nucleotides: Substrate Binding Versus Catalysis. J. Org. Chem. 2005, 70, 1919-1921.

Functional Proteomics for the Discovery of Carbohydrate-Related Enzyme Activities
Pohl, N. L.* Functional Proteomics for the Discovery of Carbohydrate-Related Enzyme Activities. Curr. Opin. Chem. Biol. 2005, 9, 76-81. (invited review)

One-Step Synthesis of Labeled Sugar Nucleotides for Protein O-GlcNAc Modification Studies by Chemical Function Analysis of an Archaeal Protein
Mizanur, R. M.; Jaipuri, F. A.; Pohl, N. L.* One-Step Synthesis of Labeled Sugar Nucleotides for Protein O-GlcNAc Modification Studies by Chemical Function Analysis of an Archaeal Protein. J. Am. Chem. Soc. 2005, 127, 836-837

Unusually Broad Substrate Tolerance of a Heat-Stable Archaeal Sugar Nucleotidyltransferase for the Synthesis of Sugar Nucleotides
Mizanur, R. M.; Zea, C. J.; Pohl, N. L.* Unusually Broad Substrate Tolerance of a Heat-Stable Archaeal Sugar Nucleotidyltransferase for the Synthesis of Sugar Nucleotides. J. Am. Chem. Soc.2004, 126, 15993-15998.

Surprising Bacterial Nucleotidyltransferase Selectivity in the Conversion of Carbaglucose-1-phosphate
Ko, K.-S.; Zea, C. J.; Pohl, N. L.* Surprising Bacterial Nucleotidyltransferase Selectivity in the Conversion of Carbaglucose-1-phosphate. J. Am. Chem. Soc. 2004, 126, 13188-13189.
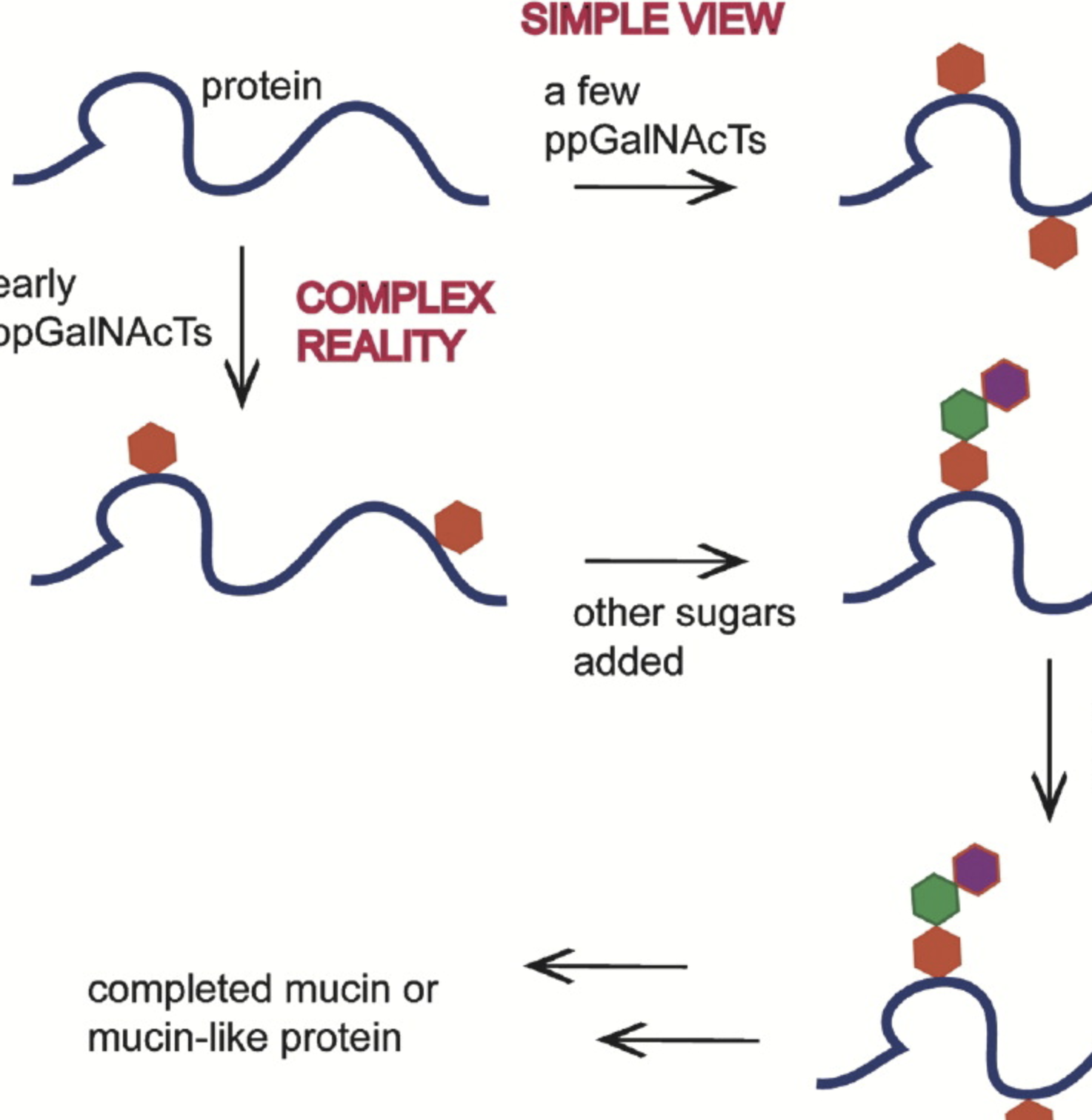
Cellular Addresses: Step One in Creating a Glycocode
Pohl, N.* Cellular Addresses: Step One in Creating a Glycocode. Chem. Biol. 2004, 11, 891-892. (invited commentary) DOI: 10.1016/j.chembiol.2004.07.004

Discovery of the Chemical Function of Glycosidases: Design, Synthesis, and Evaluation of Mass-differentiated Carbohydrate Libraries
Yu, Y.; Ko, K.-S.; Zea, C. J.; Pohl, N. L.* Discovery of the Chemical Function of Glycosidases: Design, Synthesis, and Evaluation of Mass-differentiated Carbohydrate Libraries. Org. Lett. 2004, 6, 2031-2033.
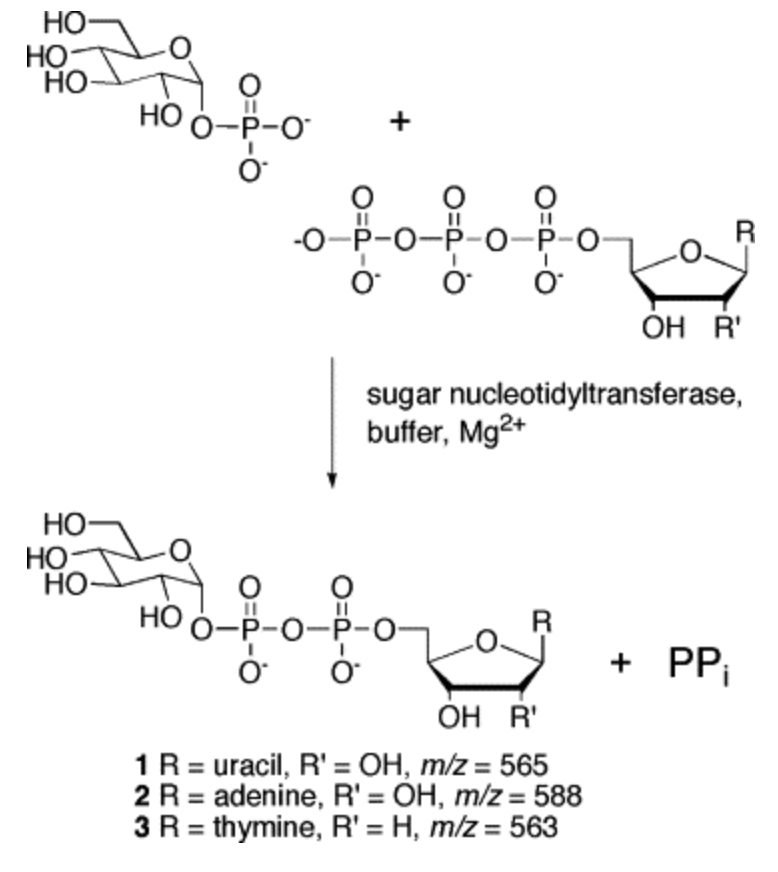
General Assay for Sugar Nucleotidyltransferases Using Electrospray Ionization Mass Spectrometry
Zea, C. J.; Pohl, N. L.* General Assay for Sugar Nucleotidyltransferases Using Electrospray Ionization Mass Spectrometry. Anal. Biochem. 2004, 328, 196-202. DOI: 10.1016/j.ab.2004.01.019
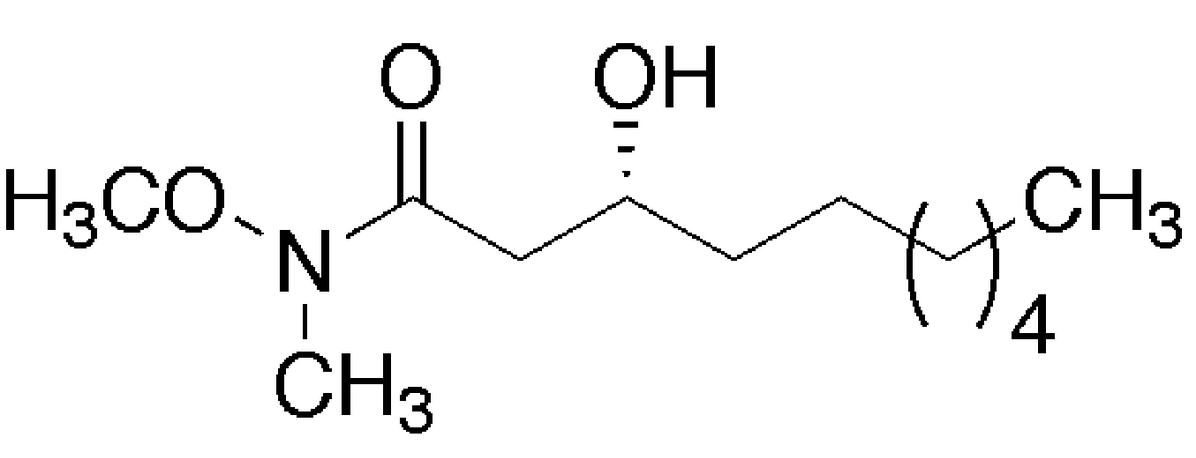
Microwave-assisted cleavage of Weinreb amide for carboxylate protection in the synthesis of a (R)-3-hydroxyalkanoic acid
Jaipuri, F. A.; Jofre, M. F.; Schwarz, K.; Pohl, N. L.* Microwave-assisted cleavage of Weinreb amide for carboxylate protection in the synthesis of a (R)-3-hydroxyalkanoic acid. Tetrahedron Lett. 2004, 45, 4149-4152. DOI: 10.1016/j.tetlet.2004.03.148
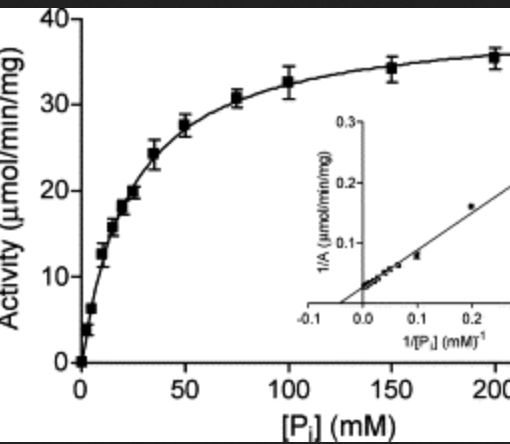
Kinetic and Substrate Binding Analysis of Phosphorylase b Via Electrospray Ionization Mass Spectrometry: A Model for Chemical Proteomics of Sugar Phosphorylases
Zea, C. J.; Pohl, N. L.* Kinetic and Substrate Binding Analysis of Phosphorylase b Via Electrospray Ionization Mass Spectrometry: A Model for Chemical Proteomics of Sugar Phosphorylases. Anal. Biochem. 2004, 327, 107-113.DOI: 10.1016/j.ab.2003.12.022
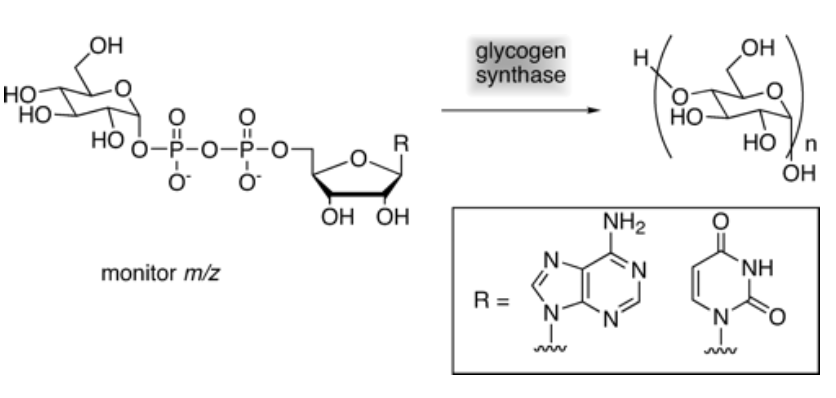
Discovery of the Archaeal Chemical Link Between Glycogen (Starch) Synthase Families Using a New Mass Spectrometry Assay
Zea, C. J.; MacDonell, S. W.; Pohl, N. L.* Discovery of the Archaeal Chemical Link Between Glycogen (Starch) Synthase Families Using a New Mass Spectrometry Assay. J. Am. Chem. Soc. 2003, 125, 13666-13667. DOI: 10.1021/ja037298o
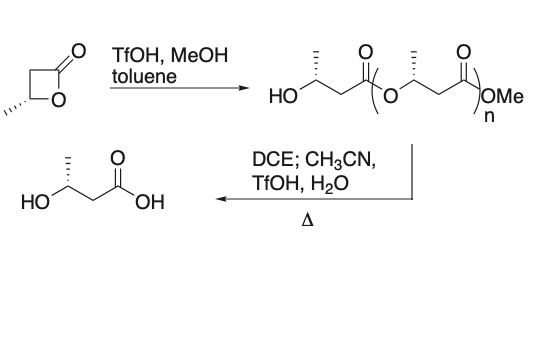
Protic Acid-Catalyzed Polymerization of Beta-Lactones for the Synthesis of Chiral Polyesters
Jaipuri, F. A.; Bower, B. D.; Pohl, N. L.* Protic Acid-Catalyzed Polymerization of Beta-Lactones for the Synthesis of Chiral Polyesters. Tetrahedron: Asymmetry 2003, 14, 3249-3252. DOI: 10.1016/j.tetasy.2003.08.025
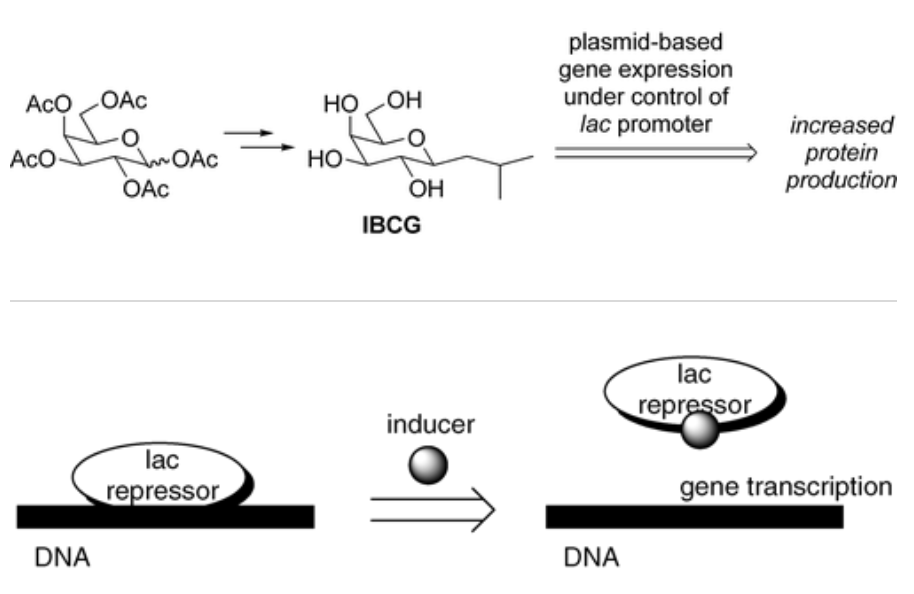
Synthesis of Isobutyl-C-galactoside (IBCG) as an Isopropylthiogalactoside (IPTG) Substitute for Increased Induction of Protein Expression
Ko, K.-S.; Kruse, J.; Pohl, N. L.* Synthesis of Isobutyl-C-galactoside (IBCG) as an Isopropylthiogalactoside (IPTG) Substitute for Increased Induction of Protein Expression. Org. Lett. 2003, 5, 1781-1783. DOI: 10.1021/ol034444m
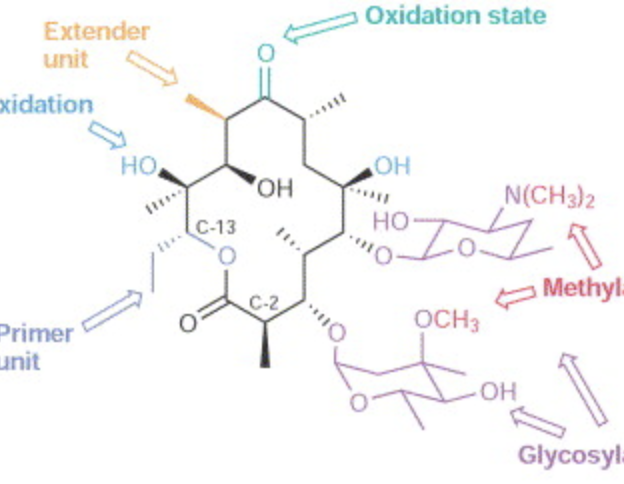
Nonnatural Substrates for Polyketide Synthases and their Associated Modifying Enzymes
Pohl, N. L.* Nonnatural Substrates for Polyketide Synthases and their Associated Modifying Enzymes. Curr. Opin. Chem. Biol. 2002, 6, 773-778. (invited review) DOI: 10.1016/S1367-5931(02)00360-5
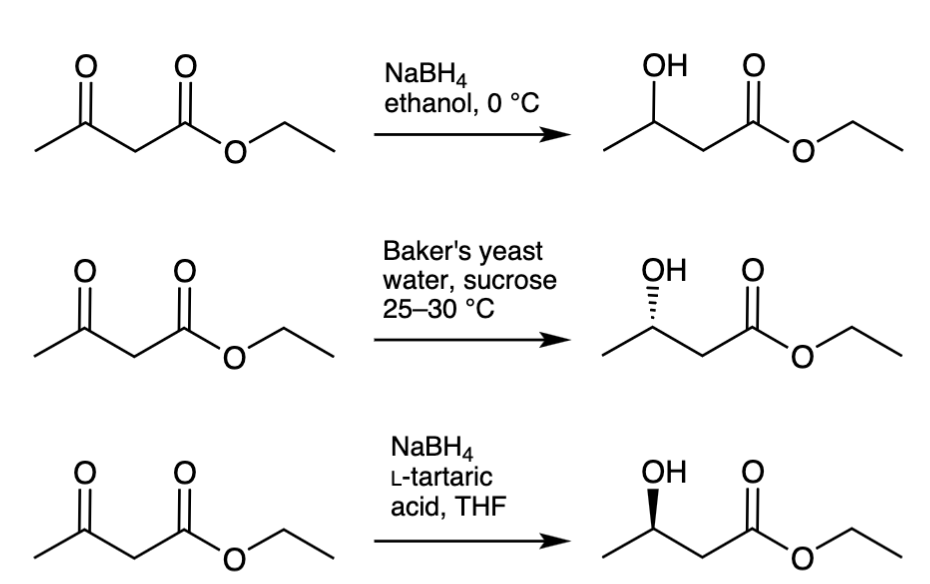
Compounds and Green Chemistry in Undergraduate Organic Laboratories: Reduction of a Ketone By Sodium Borohydride And Baker’s Yeast
Pohl, N.*; Clague. A.; Schwarz, K. Chiral Compounds and Green Chemistry in Undergraduate Organic Laboratories: Reduction of a Ketone By Sodium Borohydride And Baker’s Yeast. J. Chem. Educ.2002, 79, 727-728. DOI: 10.1021/ed079p727
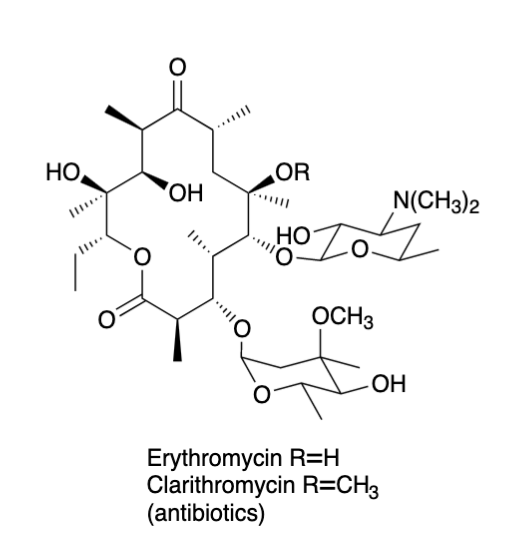
Developing New Antibiotics With Combinatorial Biosynthesis
Pohl, N. L.* Developing New Antibiotics With Combinatorial Biosynthesis. J. Chem. Educ. 2000, 77, 1421-1423. (invited review)DOI: 10.1021/ed077p1421
